Abstract
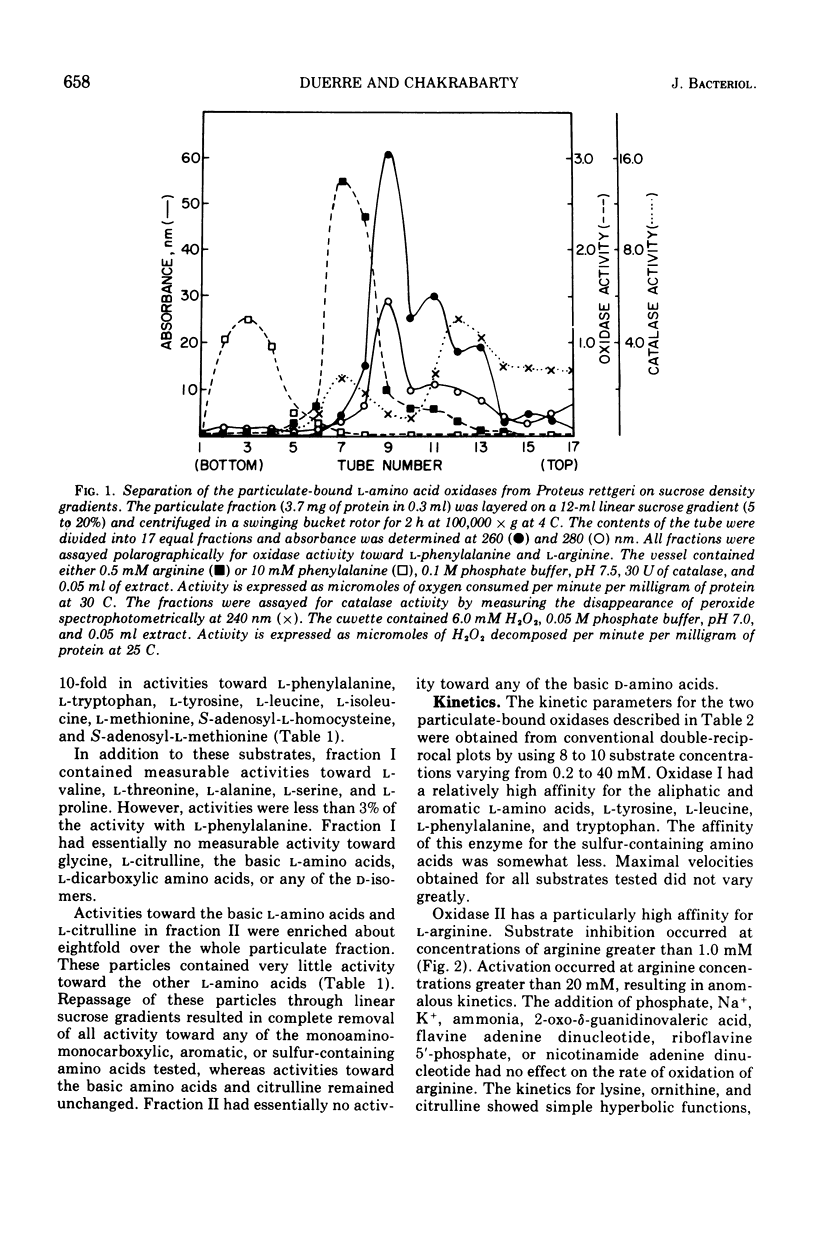
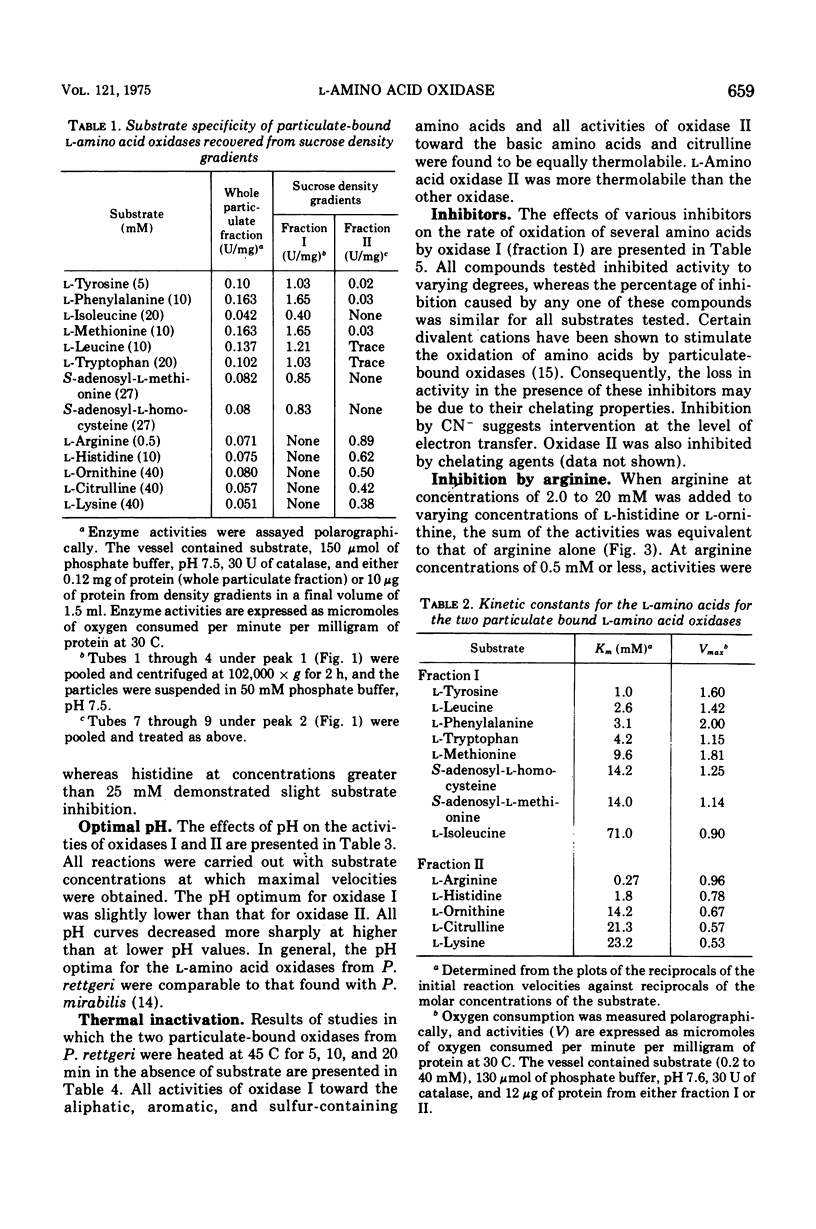
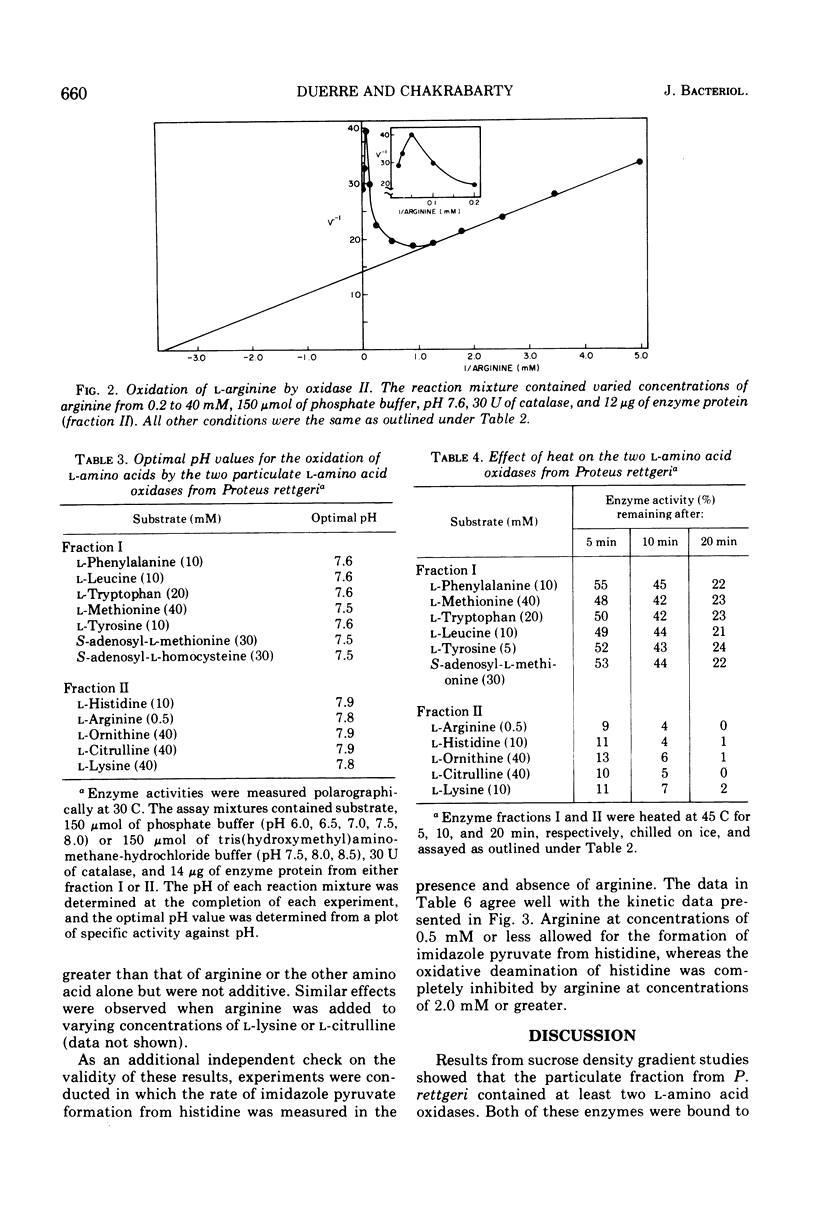
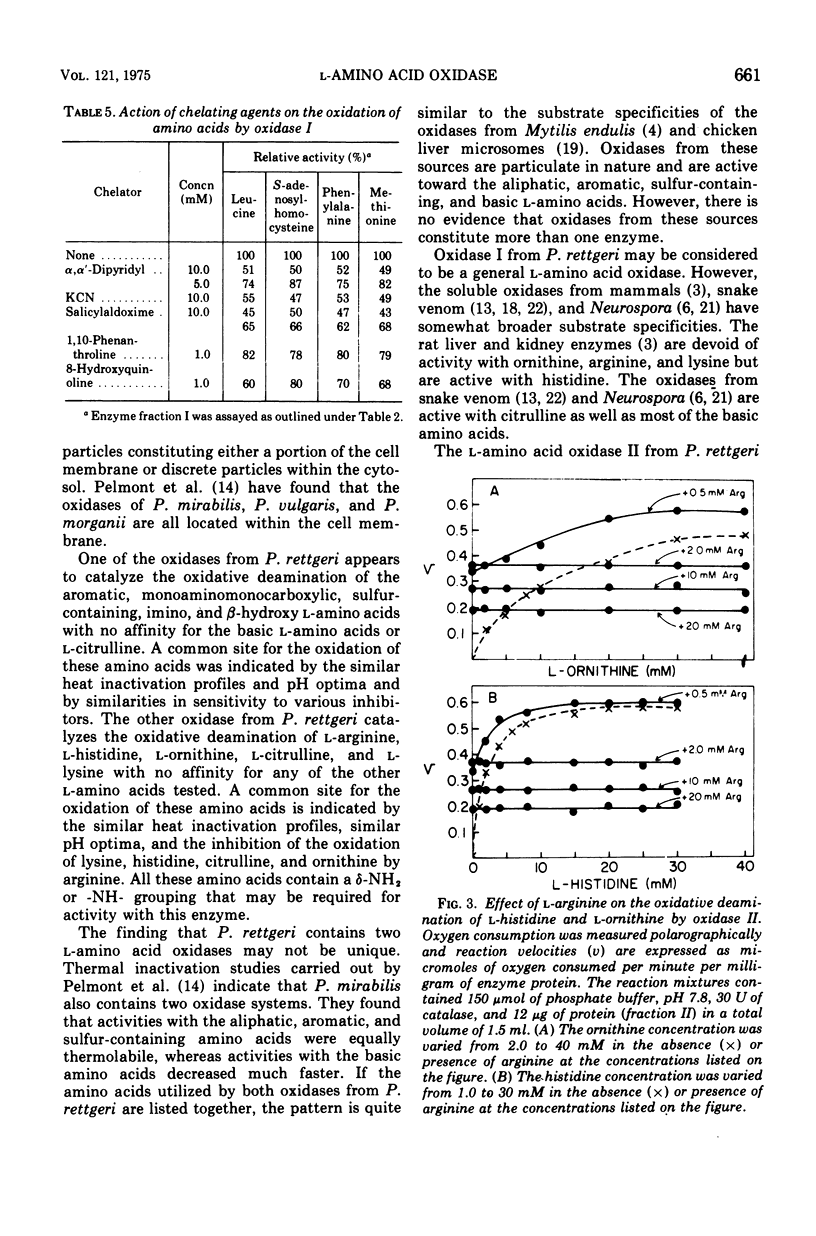
Proteus rettgeri has been found to contain two separable 1-amino acid oxidases. Both enzymes are particulate in nature, neither being ribosomal bound. One of these enzymes appears to have broad specificity, being active toward monoaminomonocarboxylic, imino, aromatic, sulfur-containing, and beta-hydroxyamino acids. The other enzyme has more limited specificity, catalyzing the oxidative deamination of the basic amino acids and citrulline. The affinity of this oxidase for the various substrates at pH 7.6 in decreasing order is arginine, histidine, ornithine, citrulline, and lysine. This enzyme has a particularly high affinity for arginine (Km equal to 0.27 mM), and anomalous kinetics are observed with increasing substrate concentrations. When concentrations of arginine greater than 1.0mM were added to the reaction containing histidine, imidazole pyruvate formation was completely inhibited.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- BLASCHKO H., HOPE D. B. The oxidation of L-amino acids by Mytilus edulis. Biochem J. 1956 Feb;62(2):335–339. doi: 10.1042/bj0620335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. The L-amino-acid oxidase of Neurospora. Biochem J. 1951 Dec;50(2):258–268. doi: 10.1042/bj0500258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. S., Walgate J. H., Duerre J. A. Oxidative deamination of sulfur amino acids by bacterial and snake venom L-amino acid oxidase. Arch Biochem Biophys. 1971 Sep;146(1):54–63. doi: 10.1016/s0003-9861(71)80040-1. [DOI] [PubMed] [Google Scholar]
- Duerre J. A., Buckley P. J. Pigment production from tryptophan by an Achromobacter species. J Bacteriol. 1965 Dec;90(6):1686–1691. doi: 10.1128/jb.90.6.1686-1691.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthi V. S., Buckley P. J., Duerre J. A. Pigment formation from L-tryptophan by a particulate fraction from an Achromobacter species. Arch Biochem Biophys. 1969 Mar;130(1):636–645. doi: 10.1016/0003-9861(69)90081-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- PAIK W. K., KIM S. PH-SUBSTRATE RELATIONSHIP OF L-AMINO ACID OXIDASES FROM SNAKE VENOM AND RAT KIDNEY. Biochim Biophys Acta. 1965 Jan;96:66–74. doi: 10.1016/0005-2787(65)90610-6. [DOI] [PubMed] [Google Scholar]
- Pelmont J., Arlaud G., Rossat A. M. L-aminoacide oxydases des enveloppes de Proteus mirabilis: propriétés générales. Biochimie. 1972;54(10):1359–1374. doi: 10.1016/s0300-9084(72)80076-2. [DOI] [PubMed] [Google Scholar]
- ROCHE J., GLAHN P. E., MANCHON P., von THOAI [On a new L-amino acid oxidase activated by magnesium]. Biochim Biophys Acta. 1959 Sep;35:111–122. doi: 10.1016/0006-3002(59)90340-3. [DOI] [PubMed] [Google Scholar]
- SCHLENK F., DEPALMA R. E. The formation of S-adenosylmethionine in yeast. J Biol Chem. 1957 Dec;229(2):1037–1050. [PubMed] [Google Scholar]
- SHAW C., CLARKE P. H. Biochemical classification of Proteus and Providence cultures. J Gen Microbiol. 1955 Aug;13(1):155–161. doi: 10.1099/00221287-13-1-155. [DOI] [PubMed] [Google Scholar]
- SINGER T. P., KEARNEY E. B. The L-amino acid oxidases of snake venom. II. Isolation and characterization of homogeneous L-amino acid oxidase. Arch Biochem. 1950 Nov;29(1):190–209. [PubMed] [Google Scholar]
- THAYER P. S., HOROWITZ N. H. The L-amino acid oxidase of Neurospora. J Biol Chem. 1951 Oct;192(2):755–767. [PubMed] [Google Scholar]


