Abstract
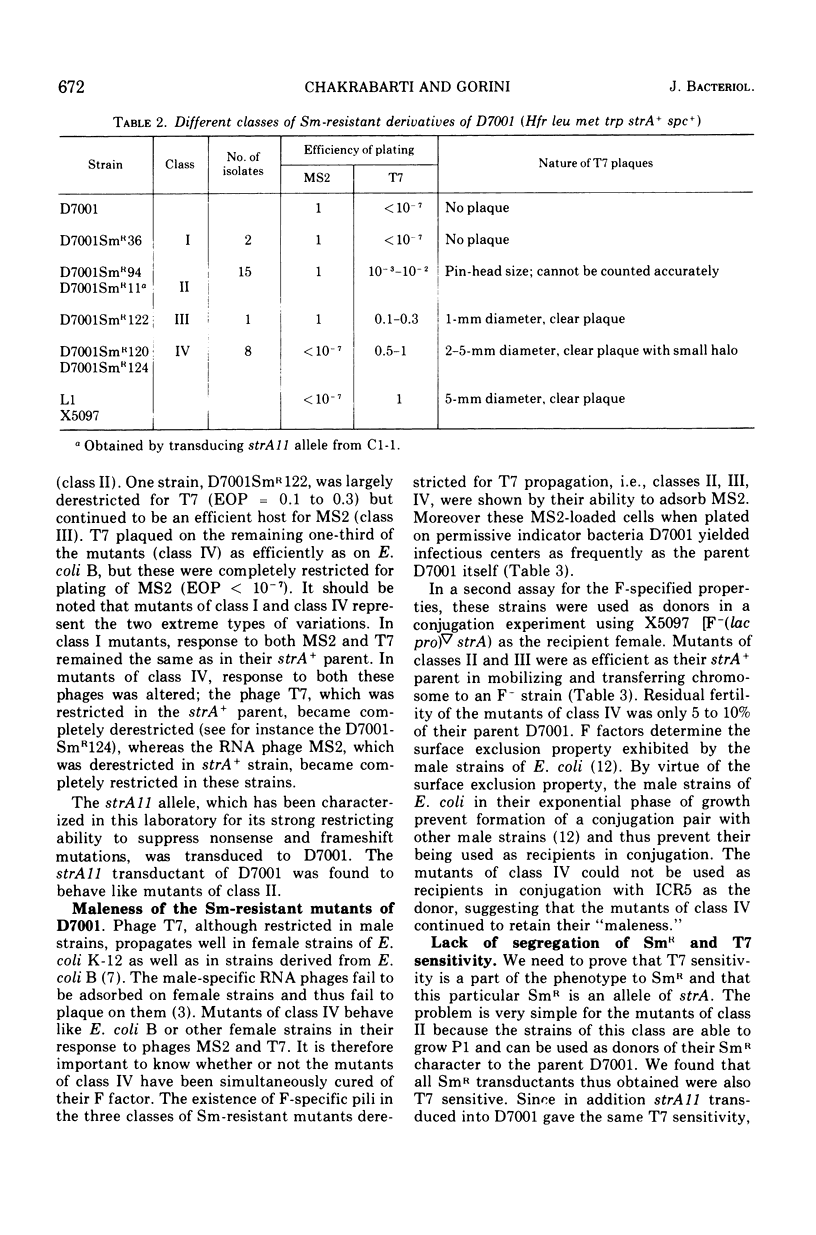
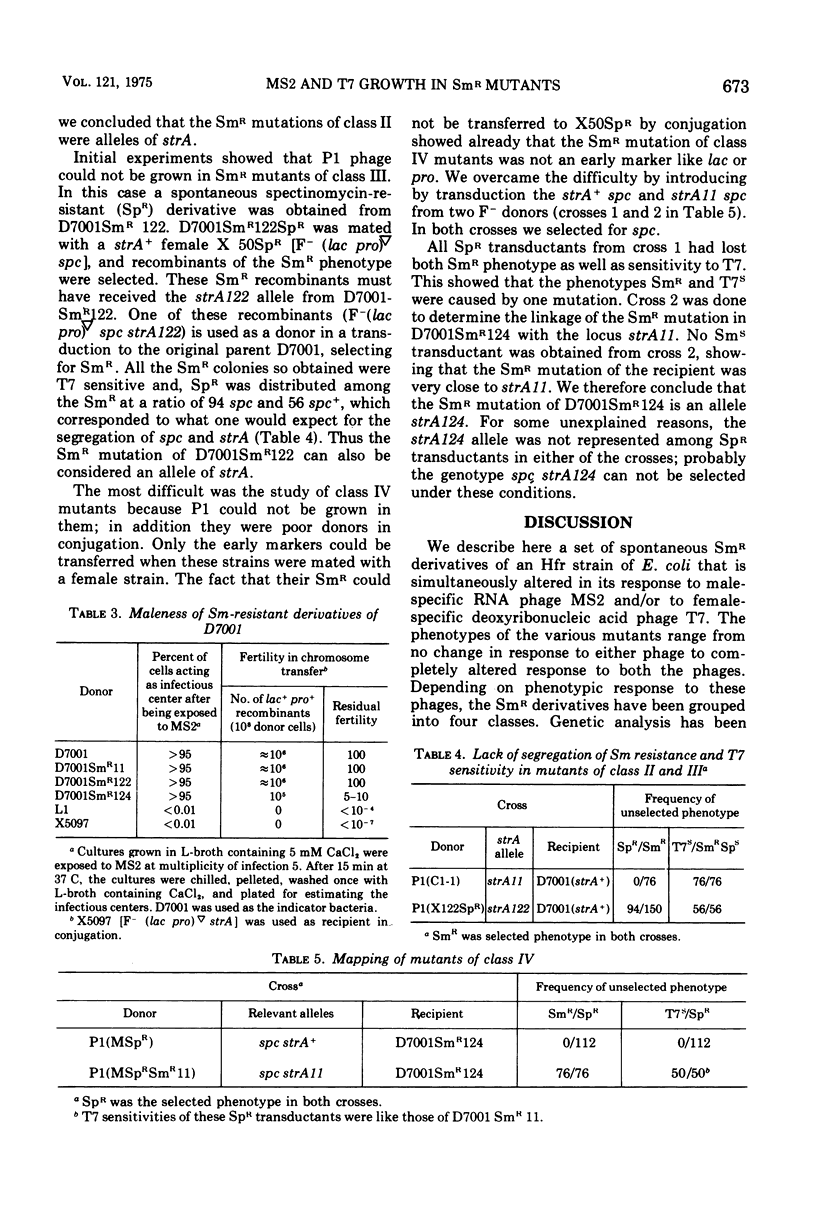
Streptomycin-resistant mutants of an Hfr strain of Escherichia coli K were examined for their ability to support the growth of male-specific ribonucleic acid phage MS2 and female-specific deoxyribonucleic acid phage T7. Normally, the Hfr strain allows propagation of MS2 and is lysed by it (efficiency of plating equal to 1), whereas the same strain restricts propagation of T7 and is not lysed by it (efficiency of plating smaller than 10-7). Twenty-four isolates out of 26 independently obtained streptomycin-resistant mutants are partially or completely derestricted for propagation of T7; efficiency of plating of T7 in such strains ranges from 10-3-1. Depending on their response to plating of MS2 and T7, the streptomycin-resistant mutants can be divided into four classes. The mutants in all four classes continue to be "male" in conjugation with F- strains. Genetic analysis is presented to show that restriction of MS2, derestriction of T7, and resistance to streptomycin are the pleiotropic effects of a single mutation at the strA locus.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumberg D. D., Malamy M. H. Evidence for the presence of nontranslated T7 late mRNA in infected F'(PIF+) episome-containing cells. J Virol. 1974 Feb;13(2):378–385. doi: 10.1128/jvi.13.2.378-385.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckenridge L., Gorini L. Genetic analysis of streptomycin resistance in Escherichia coli. Genetics. 1970 May;65(1):9–25. doi: 10.1093/genetics/65.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Caro L. G., Allison D. P., Stallions D. R. Early stages of conjugation in Escherichia coli. J Bacteriol. 1969 Nov;100(2):1091–1104. doi: 10.1128/jb.100.2.1091-1104.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorini L. Ribosomal discrimination of tRNAs. Nat New Biol. 1971 Dec 29;234(52):261–264. doi: 10.1038/newbio234261a0. [DOI] [PubMed] [Google Scholar]
- Held W. A., Gette W. R., Nomura M. Role of 16S ribosomal ribonucleic acid and the 30S ribosomal protein S12 in the initiation of natural messenger ribonucleic acid translation. Biochemistry. 1974 May 7;13(10):2115–2122. doi: 10.1021/bi00707a019. [DOI] [PubMed] [Google Scholar]
- Kang S. S. A mutant of Escherichia coli with temperature-sensitive streptomycin protein. Proc Natl Acad Sci U S A. 1970 Mar;65(3):544–550. doi: 10.1073/pnas.65.3.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Morrison T. G., Malamy M. H. T7 translational control mechanisms and their inhibiton by F factors. Nat New Biol. 1971 May 12;231(19):37–41. doi: 10.1038/newbio231037a0. [DOI] [PubMed] [Google Scholar]
- Novick R. P. Extrachromosomal inheritance in bacteria. Bacteriol Rev. 1969 Jun;33(2):210–263. doi: 10.1128/br.33.2.210-263.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheps R., Zeller H., Revel M. Deficiency in initiation factors of protein synthesis induced by phage T7 in E. coli F(+) strains. FEBS Lett. 1972 Oct 15;27(1):1–4. doi: 10.1016/0014-5793(72)80394-6. [DOI] [PubMed] [Google Scholar]


