Abstract
The several hundred members of the eukaryotic protein kinase superfamily characterized to date share a similar catalytic domain structure, consisting of 12 conserved subdomains. Here we report the existence and wide occurrence in eukaryotes of a protein kinase with a completely different structure. We cloned and sequenced the human, mouse, rat, and Caenorhabditis elegans eukaryotic elongation factor-2 kinase (eEF-2 kinase) and found that with the exception of the ATP-binding site, they do not contain any sequence motifs characteristic of the eukaryotic protein kinase superfamily. Comparison of different eEF-2 kinase sequences reveals a highly conserved region of ≈200 amino acids which was found to be homologous to the catalytic domain of the recently described myosin heavy chain kinase A (MHCK A) from Dictyostelium. This suggests that eEF-2 kinase and MHCK A are members of a new class of protein kinases with a novel catalytic domain structure.
Protein phosphorylation plays a pivotal role in a wide variety of cellular processes (1–3). Two protein kinase superfamilies have been described. The vast majority of protein kinases belong to the serine/threonine/tyrosine kinase superfamily (2, 3). Several hundred members of this superfamily have thus far been characterized and found to share similar structural organization of their catalytic domains consisting of 12 conserved subdomains (2, 3). There is also the histidine kinase superfamily consisting primarily of sensor components of the prokaryotic two-component signal transduction systems (4–6). Eukaryotic members of this superfamily have been recently described (7–9). In addition, mitochondrial branched-chain α-ketoacid dehydrogenase kinase (10) and the mitochondrial pyruvate dehydrogenase kinase (11) have been described which are structurally related to the histidine kinases, but phosphorylate their substrates on serine. Finally, the existence of several protein kinases have recently been reported which have very little or no homology to either superfamily (12–16). However, most of these unusual protein kinases are not well characterized and therefore viewed as an exception to the general rule.
Here we describe the cloning and sequencing of the extensively characterized eukaryotic elongation factor-2 kinase (eEF-2 kinase), from various eukaryotic organisms which reveals the existence of a novel class of protein kinases. eEF-2 kinase, previously known as Ca2+/calmodulin-dependent protein kinase III, is highly specific for elongation factor-2 (eEF-2), an abundant cytoplasmic protein that catalyzes the movement of the ribosome along mRNA during translation in eukaryotic cells (reviewed in refs. 17 and 18). eEF-2 kinase activity is found in virtually all mammalian tissues as well as in various invertebrate organisms (19). eEF-2 kinase phosphorylates eEF-2 at two threonine residues that are conserved in all eukaryotes and are located within a GTP-binding domain (17, 18). Phosphorylation of eEF-2 by eEF-2 kinase results in the inactivation of eEF-2 (20). Because eEF-2 kinase is Ca2+ and calmodulin-dependent, eEF-2 phosphorylation is a mechanism by which changes in the intracellular calcium concentration can modulate the rate of protein synthesis. There is also evidence that eEF-2 phosphorylation is involved in the regulation of cell cycle progression. For example, transient phosphorylation of eEF-2 occurs during the mitogenic stimulation of quiescent cells (21) and during mitosis (22). In addition, changes in the level of eEF-2 kinase activity accompany such processes as cellular differentiation (23–25), oogenesis (26), and malignant transformation (27).
Surprisingly, sequence analysis reveals that eEF-2 kinase appears to have no homology to either Ca2+/calmodulin-dependent protein kinases or to any members of the two known protein kinase superfamilies. We found that it is homologous to the recently described myosin heavy chain kinase A (MHCK A) from Dictyostelium (15) and thus these two kinases define a novel class of protein kinases that may represent a new superfamily.
MATERIALS AND METHODS
Materials.
Buffer reagents were obtained from Sigma. All radioisotopes were from Amersham with the exception of [35S]methionine, which was from DuPont/NEN. PCR reagents and restriction enzymes were from GIBCO/BRL. TA cloning kits and cloning vectors were from Invitrogen. Mammalian cDNA libraries were from CLONTECH, and the TnT in vitro transcription/translation kits were from Promega.
Rabbit reticulocyte eEF-2 and eEF-2 kinase were purified as described (28).
Peptide Sequencing.
Peptides were generated from the nitrocellulose-bound 103-kDa eEF-2 kinase protein by in situ tryptic digestion (29) and fractionated by reverse-phase HPLC (30) using a 1.0 mm Reliasil C18 column. Selected peak fractions were then analyzed by a combination of automated Edman sequencing and matrix-assisted laser-desorption time-of-flight mass spectrometry (29).
cDNA Cloning.
To clone the cDNA for C. elegans eEF-2 kinase, oligonucleotide primers were designed based on the amino and carboxy termini of the predicted gene product from F42A10.4. Reverse transcriptase–PCR (RT-PCR) was performed using these primers and total RNA from C. elegans (a gift from Monica Driscoll, Rutgers University). A single PCR product of ≈2.3 kb was obtained and gel-purified using a gel extraction kit (Qiagen, Chatsworth, CA). The fragment was ligated into vector pCR2.1 using the TA cloning kit (Invitrogen), and then transformed into Escherichia coli. Plasmid DNA was purified, and restriction analysis used to verify the orientation of the coding sequence with respect to the T7 promoter. Two clones (Cefk-1 and Cefk-2, C. elegans eEF-2 kinase isoforms 1 and 2) were chosen and sequenced using a Li-Cor (Lincoln, NE) Long Read IR model 400L Automated DNA Sequencer. Analysis revealed that the two clones were identical except for a deletion of 24 bp in Cefk-2 which corresponds to exon 4 and probably represents an alternatively spliced form.
To clone the mouse eEF-2 kinase, degenerate primers were designed based on the amino acid sequence of two peptides from rabbit eEF-2 kinase (LTPQAFSHFTFER and LANXYYEKAE): primer A, CA(G/A)GC(C/G/T/A)TT(C/T)(T/A)(C/G)(T/CCA(C/T)TT(C/T)AC(C/G/T/A)TT(C/T)GA(G/A)(C/A)G; and primer B, TC(C/G/T/A)GC(C/T)TT(C/T)TC(G/A)TA(G/A)TA(C/T)TT(G/A)TT(C/G/A/T)GC. RT-PCR was performed using primers A and B and poly(A)+ RNA from mouse spleen (CLONTECH). A single PCR product (≈1.6 kb) was cloned into pCR2.1 (Invitrogen) and sequenced. Using sequence information from these mouse eEF-2 kinase cDNA fragments, new primers were designed for 5′ rapid amplification of cDNA ends (RACE) and 3′ RACE to obtain full-length mouse eEF-2 kinase cDNA. 5′ RACE and 3′ RACE were performed using Marathon-Ready mouse spleen cDNA (CLONTECH). This was carried out according to the manufacturer’s instructions using the primers AP1 and C (TACAATCAGCTGATGACCAGAACGCTC) 5′ antisense, or D (GGATTTGGACTGGACAAGAACCCCC) 3′ sense.
To clone rat eEF-2 kinase, PCR was performed on a rat PC12 cDNA library cloned in λGT10 (CLONTECH) using primer B and vector primers. A 700-bp fragment was specifically amplified. The fragment was cloned into pCR2.1 (Invitrogen) and sequenced. This 700-bp fragment was radiolabeled and used to probe the same PC12 cDNA library (600,000 plaques). Fourteen positives were obtained in the initial screening. Five plaques were chosen for further analysis and sequencing based on insert sizes that ranged from 1.4 to 2.0 kb.
Expression of eEF-2 Kinase in Cell-Free System.
Plasmid DNA from clones Cefk-1, Cefk-2, as well as mouse and human eEF-2 kinase cDNA were used in the TnT wheat germ extract coupled transcription/translation system (Promega). [35S]Methionine-labeled products were then analyzed by SDS/PAGE. The reaction mixture (50 μl total volume) contained 1 μg of plasmid DNA and 26 μCi of [35S]methionine (specific activity = 1175.0 Ci/mmol; 1 Ci = 37 GBq). Other components were added to the reaction mixture according to the manufacturer’s protocol. The reaction mixture was incubated for 1.5 h at 30°C and terminated by incubation on ice. A 10 μl aliquot of the reaction mixture was mixed with 2 μl of 5× Laemmli buffer and boiled for 5 min. Samples were analyzed by SDS/PAGE on 8% gels and autoradiography.
eEF-2 Kinase Assay.
To assay for eEF-2 kinase activity, 5 μl from each fraction was added to a reaction mixture (40 μl) containing 50 mM Hepes-KOH (pH 7.4), 10 mM magnesium acetate, 0.1 mM CaCl2, 5 mM dithiothreitol, 50 μM ATP, 2 μCi [γ-32P]ATP, 0.6 μg calmodulin, and 0.5 μg rabbit reticulocyte eEF-2. Reactions were incubated at 30°C for 2 min and were terminated by adding 20 μl of 3× Laemmli sample buffer. Samples were boiled for 5 min and proteins separated by SDS/PAGE on 8% gels. Phosphoproteins were analyzed by autoradiography.
Chromatography on Mono Q Fast Protein Liquid Chromatography Column.
The transcription/translation reaction was diluted 4-fold with buffer A (20 mM Tris⋅HCl, pH 7.4/1 mM MgCl2/10% glycerol/7 mM 2-mercaptoethanol) and applied to a HR5/5 Mono Q column (Pharmacia) equilibrated with buffer A. The column was developed with 20 column volumes of a 50–600 mM KCl linear gradient in buffer A.
Northern Blot Analysis.
eEF-2 kinase and eEF-2 hybridizations were performed using a 1.6-kb EcoRI mouse cDNA fragment and a 2.6-kb EcoRI human cDNA fragment, respectively. cDNAs were labeled with [32P]dCTP using the random-primed DNA labeling method (31). A multiple tissue Northern blot (CLONTECH) was prehybridized at 42°C for 16 h in a 50% formamide solution containing 10× Denhardt’s, 5× SSPE, 2% SDS, and 100 μg/ml salmon sperm DNA. Hybridizations were completed in the same solution containing the 32P-labeled probe (1 × 106 cpm/ml; specific activity, ≈1 × 108 dpm/μg DNA) and 10% dextran sulfate at 42°C for 16 h. Blots were washed twice at room temperature (15 min) in 2× SSPE, 0.05% SDS, and once at 50°C (15 min) in 0.5× SSPE, 0.5% SDS. RNA/cDNA hybrids were visualized by autoradiography.
Preparation of C. elegans Extracts.
Extracts from C. elegans were prepared as follows: wild type (N2) C. elegans were grown on bacterial strain OP50 spread on nematode growth medium according to ref. 32. Worms from twenty-seven 100-mm culture plates were harvested by trituration with cold PBS, pelleted by gentle centrifugation, and resuspended in 2.0 ml of 25 mM Hepes (pH 7.4), 100 mM NaCl, 20 mM sodium pyrophosphate, 3 mM EGTA, 0.5 mM phenylmethylsulfonyl fluoride, 1.25 μg/ml leupeptin, and 1.25 μg/ml pepstatin A. The suspension was homogenized by mechanical shearing in a Dounce homogenizer, and centrifuged at 16,000 × g for 30 min at 4°C. The protein concentration of the supernatant was determined by Bio-Rad protein assay.
RESULTS AND DISCUSSION
Molecular Cloning of C. elegans eEF-2 Kinase cDNA.
Recently we purified eEF-2 kinase from rabbit reticulocyte lysate to near homogeneity (28) which enabled us to determine its partial amino acid sequence (see Materials and Methods). Two peptide sequences (LTPQAFSHFTFER and LANXYYEKAE) were compared with entries in a nonredundant database using the National Center for Biotechnology Information blast program (33). Matches were found with a C. elegans hypothetical protein (F42A10.4; GenBank accession number U10414U10414). This sequence was obtained from the C. elegans genome sequencing project and is located on chromosome III (34). The 100% identity between the sequenced peptides and the C. elegans protein, as well as the fact that the predicted molecular weight of the C. elegans protein is similar to that of eEF-2 kinase, suggested that this gene encoded eEF-2 kinase. We cloned the full-length cDNA by RT-PCR using C. elegans total RNA. Several clones were isolated and sequenced. Cefk-1 has six of the predicted exons and encodes 768 amino acids. Cefk-2 represents an alternatively spliced form that has five exons; it is missing amino acids 625–632 that correspond to exon four.
Expression of C. elegans eEF-2 Kinase in a Cell-Free Coupled Transcription/Translation System and Analysis of Kinase Activity.
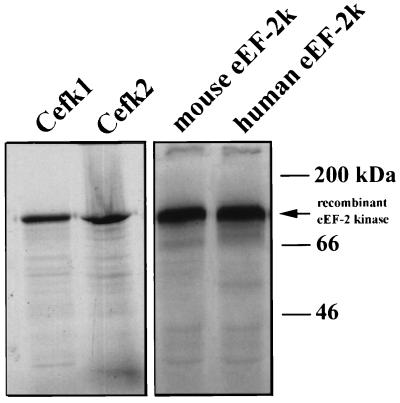
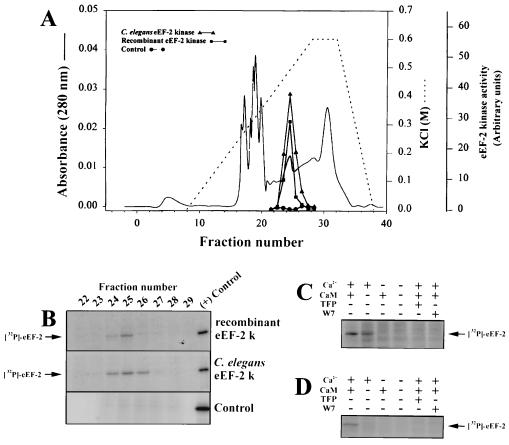
To determine whether Cefk-1 and Cefk-2 have eEF-2 kinase activity, we expressed them in a cell-free coupled transcription/translation system. Translation of Cefk-1 and Cefk-2 produced products with an apparent molecular weight of 100 kDa (Fig. 1), which is slightly larger than the computer-predicted molecular weight of the protein but is identical to the molecular weight of rabbit reticulocyte eEF-2 kinase as determined by SDS/PAGE. The translation products of the mixture of Cefk-1 and Cefk-2 are able to phosphorylate eEF-2 (Fig. 2) and elute from a Mono Q column at the same position as endogenous C. elegans eEF-2 kinase (Fig. 2A). The eEF-2 phosphorylation activity of the recombinant protein is Ca2+/calmodulin-dependent (Fig. 2C). We are currently studying whether there are differences in the catalytic properties between Cefk-1 and Cefk-2 isoforms.
Figure 1.
Expression of recombinant eEF-2 kinase in vitro. Plasmid DNA from clones Cefk-1, Cefk-2, as well as mouse and human eEF-2 kinase cDNA were used in the TnT wheat germ extract coupled transcription/translation system (Promega). [35S]Methionine-labeled products were then analyzed by SDS/PAGE.
Figure 2.
Activity of recombinant eEF-2 kinase in vitro. A large-scale (0.5 ml) reaction using a mixture of Cefk-1 and Cefk-2 plasmids was run as in Fig. 1, with the omission of labeled methionine. In the control experiment, the reaction was run with a plasmid containing a luciferase gene. (A) The reaction mixtures were separated by chromatography on a Mono Q column as described. (B) eEF-2 kinase activity in fractions was measured as the ability to phosphorylate purified rabbit eEF-2 in the presence of [γ-32P]ATP. Purified rabbit reticulocyte eEF-2 kinase was used in the (+) control experiments. (C) Ca2+/calmodulin-dependency of recombinant C. elegans eEF-2 kinase. Mono Q fraction 25 was assayed in a standard eEF-2 kinase assay in the presence and absence of Ca2+ and calmodulin and 20 μM trifluoperazine (TFP) or N-(6-aminohexyl)-5-chloro-1-napthalene-sulfonamide (W7). (D) Ca2+/calmodulin-dependency of recombinant human eEF-2 kinase. Human eEF-2 kinase cDNA was expressed in a coupled transcription/translation system as described above and eEF-2 kinase activity was assayed without further purification.
Molecular Cloning of cDNAs Encoding Mouse, Rat, and Human eEF-2 Kinases.
To determine the amino acid sequence of mammalian eEF-2 kinase, we cloned and sequenced the cDNA of mouse eEF-2 kinase. We reasoned that since the sequenced peptides from rabbit eEF-2 kinase were 100% identical to C. elegans eEF-2 kinase, then the two peptides should also match the sequence of mouse eEF-2 kinase. Degenerate primers were designed based on the amino acid sequence of the peptides and were used to perform RT-PCR on mouse spleen poly(A)+ mRNA. A single PCR product of ≈1.6 kb was obtained and sequenced. To obtain the full-length cDNA, 5′ RACE and 3′ RACE were performed using mouse spleen cDNA. The full-length cDNA, which encodes 724 amino acids, was expressed in a cell-free coupled transcription/translation system. A single translation product with an apparent molecular weight of 100 kDa was obtained (Fig. 1).
We next cloned and sequenced cDNA for rat eEF-2 kinase using a fragment of mouse eEF-2 kinase cDNA to probe a PC12 cDNA library. While our manuscript was in preparation, a paper describing the cloning of eEF-2 kinase from rat skeletal muscle was published (35) and the reported sequence appears to be identical to the eEF-2 kinase sequence from PC12 cells. Like the mouse eEF-2 kinase, the rat eEF-2 kinase cDNA encodes a 724-amino acid protein.
We also cloned the human eEF-2 kinase cDNA. RT-PCR was performed on poly(A)+ mRNA from the human glioma cell line T98G using 20-mer primers corresponding to the 5′ and 3′ ends of the mouse eEF-2 kinase coding region. The human eEF-2 kinase cDNA encodes a 725-amino acid protein.
Expression of Mammalian eEF-2 Kinase in a Cell-Free Coupled Transcription/Translation System and Analysis of Kinase Activity.
Mouse and human eEF-2 kinase cDNAs were expressed in a coupled transcription/translation system and a product of ≈100 kDa was obtained (Fig. 1). As shown in Fig. 2, the recombinant human eEF-2 kinase activity was strictly Ca2+/calmodulin-dependent. The kinase activity was completely inhibited by the calmodulin antagonists trifluoperazine and N-(6-aminohexyl)-5-chloro-1-napthalene-sulfonamide. We have recently expressed human eEF-2 kinase in bacteria as a glutathione S-transferase fusion protein and demonstrated that the ability of the recombinant enzyme to phosphorylate eEF-2 and to undergo autophosphorylation are strictly calmodulin-dependent (data not shown).
Analysis of Mouse eEF-2 Kinase mRNA Expression in Various Tissues.
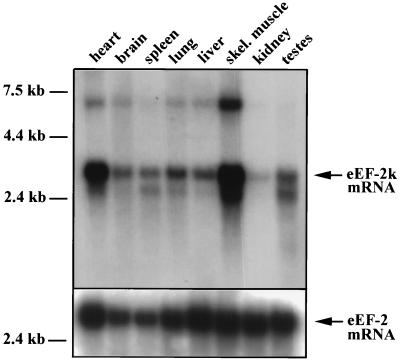
Northern blot analysis shows that eEF-2 kinase is ubiquitously expressed in mouse tissues and is particularly abundant in skeletal muscle and heart (Fig. 3). The abundance of eEF-2 kinase mRNA in muscle tissues may indicate that phosphorylation of eEF-2 is particularly important in muscle, or that there are additional substrates of eEF-2 kinase which are muscle-specific.
Figure 3.
Northern blot analysis of tissue distribution of mouse eEF-2 kinase mRNA. Northern blots of mouse tissues containing 2 μg of polyadenylylated RNA per lane were probed with the random-primed 32P-labeled mouse eEF-2 kinase cDNA (31). The major transcript appeared at 3.1 kb and minor transcripts at 6.1 and 2.5 kb were also apparent (exposure time, 5 days). The same blots were stripped and rehybridized with a human eEF-2 cDNA (exposure time, 4 days).
Lack of Homology of eEF-2 Kinase to Members of Eukaryotic Protein Kinase Superfamily.
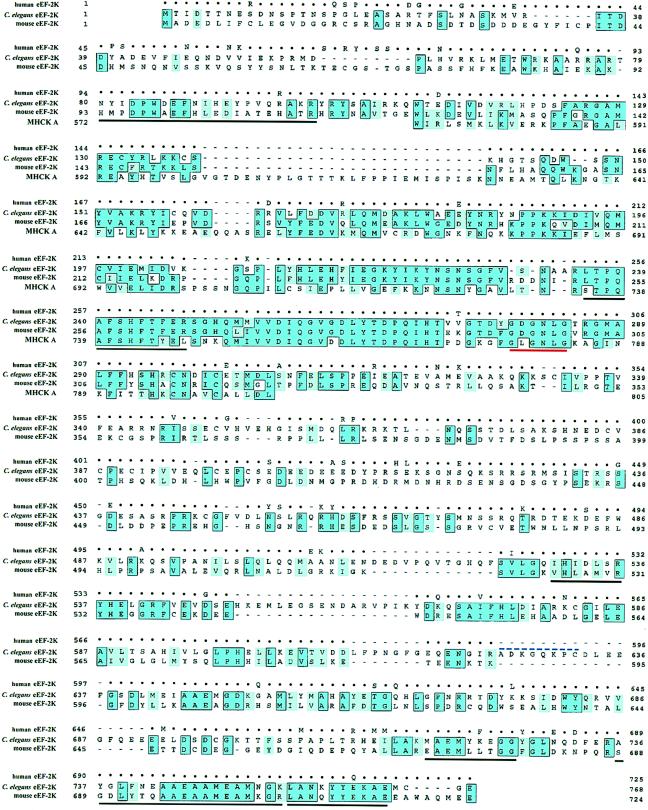
The alignment of the amino acid sequences of C. elegans and mammalian eEF-2 kinases is shown in Fig. 4. Rat and mouse eEF-2 kinase are very similar, being 97% identical and differing by only 23 amino acids. Human eEF-2 kinase is 90% identical to mouse and rat eEF-2 kinase. In contrast, C. elegans eEF-2 kinase is found to be only 40% identical to mammalian eEF-2 kinase.
Figure 4.
Sequence alignment of C. elegans, mouse, human eEF-2 kinase, and the catalytic domain of Dictyostelium discoideum MHCK A. Identical amino acids are indicated by dark blue boxed regions and chemically conserved amino acids are indicated by light blue shaded regions. Amino acids in the human sequence that are identical to the mouse sequence are represented by dots. Amino acids underlined in black correspond to the six regions that match peptides obtained from the sequencing of purified rabbit reticulocyte eEF-2 kinase. The GXGXXG nucleotide-binding motif is underlined in red. The blue dashed line over residues 625–632 in C. elegans eEF-2 kinase designates the amino acids corresponding to exon 4, which is missing in Cefk-2.
According to the current classification, eEF-2 kinase belongs to the family of closely related calmodulin-dependent protein kinases. Surprisingly, upon analyzing eEF-2 kinase sequences, we did not find any homology to the other calmodulin-dependent kinases or to any other members of the protein kinase superfamily. The only motif which it shares with all other protein kinases is the GXGXXG motif (279–284 in C. elegans eEF-2 kinase; 295–300 in mouse eEF-2 kinase) which forms a glycine-rich loop and is part of the ATP-binding site. Comparison of mammalian and C. elegans eEF-2 kinase revealed only one extended region of homology that spans ≈200 amino acids upstream of the GXGXXG motif. The high degree of similarity and the proximity to the nucleotide-binding site suggests that these 200 amino acids represent the catalytic domain. This region has a high degree of similarity and a portion of this region (amino acids 251–300 in mouse eEF-2 kinase) displays 75% identity to the catalytic domain of MHCK A (see below), which also suggests that this is the catalytic domain. In the recently published rat eEF-2 kinase sequence (35), the catalytic domain was predicted to reside between amino acids 288 and 554 based on the homology with the catalytic domain of cAMP-dependent protein kinase (PKA). Our results demonstrate that their prediction cannot be correct for several reasons. First, we find that the homology of this region with PKA is not statistically significant. Second, this region is the least conserved between mammalian and C. elegans eEF-2 kinase. Finally, according to secondary structure predictions [made by Alexei V. Finkelstein, Institute of Protein Research, Russia using the alb-globule program (36)], this region most likely has a distorted structure and contains almost no α-helices or β-strands, which are characteristic of a catalytic domain.
Because eEF-2 kinase is Ca2+/calmodulin-dependent, it should contain a calmodulin-binding domain, which is usually represented by an amphipathic α-helix. There are several regions that could possibly assume an amphipathic α-helical conformation. Further biochemical analysis is required to determine which of these is the calmodulin-binding domain.
In the C-terminal region, there is a short stretch of 22 amino acids which is 86% identical between mammalian and C. elegans eEF-2 kinase and is preceded by a longer region of weak homology. We do not know the function of this conserved region at present. One of the possibilities is that it is involved in oligomerization of the kinase. It was thought previously that eEF-2 kinase was an elongated monomer because it migrated during gel filtration as an ≈150-kDa protein and migrated on SDS gels as a 105-kDa polypeptide (17, 18). However, the molecular weight of a monomer of mammalian eEF-2 kinase based on the predicted sequence is just 82 kDa. Thus, it is possible that eEF-2 kinase is not a monomer but a dimer and that the conserved C-terminal region may be responsible for dimerization. Interestingly, according to computer prediction using the coil program, this conserved region can form a coiled-coil. Formation of coiled-coils is often responsible for dimerization (37).
Striking Homology Between eEF-2 Kinase and MHCK A from Dictyostelium.
We found that eEF-2 kinase is homologous to the central portion of the recently described MHCK A from Dictyostelium (ref. 15; see Fig. 4). This kinase was biochemically identified as a 130-kDa protein and has a demonstrated role in myosin assembly, both in vitro and in vivo (15). As with eEF-2 kinase, MHCK A displays no region with detectable similarity to the conserved catalytic domains found in known eukaryotic protein kinases. Primary structure analysis of MHCK A revealed an amino-terminal domain with a probable coiled-coil structure, a central nonrepetitive domain, and a C-terminal domain consisting of seven WD repeats (15). A fragment of the central nonrepetitive domain of MHCK A containing amino acids 552–841 was recently shown to represent the catalytic domain (38).
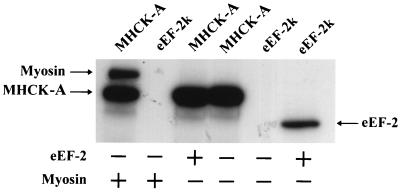
Because the catalytic domain of MHCK A and eEF-2 kinase have a high degree of similarity, the substrate specificity of these two kinases was assayed. Fig. 5 shows that MHCK A cannot phosphorylate eEF-2, and likewise, rabbit eEF-2 kinase cannot use myosin heavy chains as a substrate. This demonstrates that each of these kinases is specific for their respective substrates.
Figure 5.
Substrate specificity of eEF-2 kinase and MHCK A. Phosphorylation assays containing eEF-2 kinase (≈50 ng) or MHCK A (0.2 μg) and either 0.5 μg rabbit reticulocyte eEF-2 or 0.1 μg Dictyostelium myosin were performed under standard conditions except that incubation time was extended to 10 min.
eEF-2 Kinase and MHCK A Define a New Class of Protein Kinases.
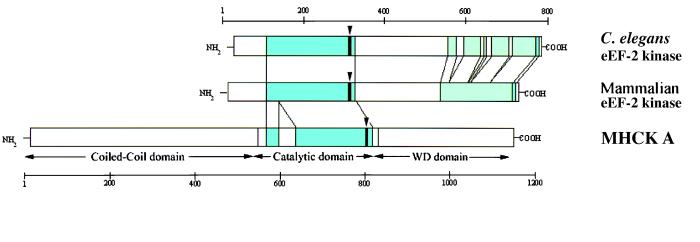
Members of the eukaryotic protein kinase superfamily are characterized by a conserved catalytic domain containing approximately 260 amino acids and is divided into twelve subdomains (2, 3, 39, 40). The three-dimensional structure of several protein kinases revealed that the catalytic domain consists of two lobes. The smaller N-terminal lobe, which has a twisted β-sheet structure, represents the ATP-binding domain. The larger C-terminal lobe, which is predominantly α-helical, is involved in substrate binding. At the primary structure level, the only motif similar between eEF-2 kinase, MHCK A, and other protein kinases is the GXGXXG motif which forms the loop interacting directly with the phosphates of ATP (2, 3, 39). In eukaryotic protein kinases, this motif is located at the very N terminus of the ATP-binding lobe of the catalytic domain. In contrast, in eEF-2 kinase and MHCK A, this motif is close to the C terminus of the catalytic domain (see Fig. 6). However, the overall topology of the ATP-binding subdomain of eEF-2 kinase and MHCK A can be similar to other protein kinases because the region upstream of the GXGXXG motif is strongly predicted to contain four or five β-strands and thus can form a twisted β-sheet.
Figure 6.
Schematic representation of the structure of mammalian and C. elegans eEF-2 kinase and MHCK A. The homologous regions are represented by blue shading. The regions of weak similarity are represented by green shading. The position of the GXGXXG motif is indicated by vertical arrows.
However, the mechanism of ATP-binding to eEF-2 kinase is probably quite different in comparison to other conventional members of the eukaryotic protein kinase superfamily. In protein kinases, there is a conserved lysine residue, corresponding to Lys-72 in cAMP-dependent protein kinase which binds to the β- and γ-phosphates of ATP and is located at about 20 amino acids downstream of the GXGXXG motif. Analysis of eEF-2 kinase and MHCK A sequences revealed that there are no conserved lysine residues in the vicinity of the GXGXXG motif. There is another atypical protein kinase, BCR-ABL, which does not contain this conserved lysine and it is proposed that it interacts with ATP via two cysteine residues (12). Interestingly, eEF-2 kinase and MHCK-A contain two conserved cysteine residues (Cys-313 and Cys-317 in mouse eEF-2 kinase) which are located near the GXGXXG motif and therefore might be involved in ATP binding. Thus the mechanism of ATP-binding of eEF-2 kinase and MHCK A is different from other members of the protein kinase superfamily, but may be similar to that of the BCR-ABL protein kinase.
The overall catalytic mechanism of eEF-2 kinase and MHCK A is probably also very different from other eukaryotic protein kinases. All members of the eukaryotic protein kinase superfamily contain a DXXXXN motif in the catalytic loop and a DFG motif in the activation segment (2, 3, 39, 40). These two motifs, which are directly involved in the catalysis of the protein phosphorylation reaction, are absent from the eEF-2 kinase and MHCK A catalytic domain.
We do not know at the present time whether there are other protein kinases which are structurally similar to eEF-2 kinase and MHCK A. An extensive search of the entire nonrestricted database of the National Center for Biotechnology Information using the blast program did not reveal any protein with a significant homology to the catalytic domain of eEF-2 kinase and MHCK A. A search of the Expressed Sequence Tag (EST) database revealed several ESTs from C. elegans, mouse and human which are essentially identical to portions of eEF-2 kinase cDNA sequences reported here. Interestingly, a search of the recently completed genome database of Saccharomyces cerevisiae did not reveal any protein with homology to eEF-2 kinase despite the fact that eEF-2 phosphorylation was reported in yeast (41).
Conclusions.
Since the catalytic domains of eEF-2 kinase and MHCK A do not share homology with other known protein kinases, these two protein kinases establish the presence of a novel and widespread superfamily of eukaryotic protein kinases. Although the existence of several unusual protein kinases have been reported, to our knowledge, we demonstrate for the first time the existence of a biochemically well-characterized and ubiquitous protein kinase that is structurally unrelated to other serine/threonine/tyrosine kinases. Contrary to the widely accepted belief that all eukaryotic protein kinases evolved from a single ancestor, our results suggest that eukaryotic protein kinases appeared at least twice during the course of evolution. This also suggests that, in addition to the relatively well-characterized catalytic mechanism employed by members of eukaryotic serine/threonine/tyrosine protein kinase superfamily, there exists another mechanism of protein phosphorylation. Further studies will reveal the molecular details of this mechanism and whether there are other protein kinases that phosphorylate their substrates using this mechanism.
Acknowledgments
We are grateful to Tom Egelhoff for providing Dictyostelium MHCK A and myosin and for sharing data prior to publication, to Monica Driscoll for the gift of C. elegans RNA and advice, and to William Wadsworth for help and advice in cultivating C. elegans and to Alexei Finkelstein for the secondary structure prediction. We thank Lynne Lacomis and Mary Lui for expert assistance with protein structural analysis. We would also like to thank Regina Felder and Tony Li for help in DNA sequencing. We are also grateful to Daniel Levin for helpful discussion. This work is supported by American Cancer Society Grant CB-147 (to A.G.R.), National Institutes of Health Grant CA 48883 (to W.N.H.), National Science Foundation Grant BIR-9420123 (to P.T.), and National Cancer Institute Core Grant 5 P30 CA08748 (to the Memorial Sloan–Kettering Cancer Center Sequencing Laboratory).
ABBREVIATIONS
- eEF-2
eukaryotic elongation factor-2
- eEF-2 kinase
eukaryotic elongation factor-2 kinase
- MHCK A
myosin heavy chain kinase A
- RT-PCR
reverse transcriptase–PCR
- Cefk-1 and Cefk-2
Caenorhabditis elegans eEF-2 kinase isoforms 1 and 2
- RACE
rapid amplification of cDNA ends
Footnotes
References
- 1.Krebs E G. Trends Biochem Sci. 1994;19:439. doi: 10.1016/0968-0004(94)90125-2. [DOI] [PubMed] [Google Scholar]
- 2.Hanks S K, Hunter T. FASEB J. 1996;9:576–596. [PubMed] [Google Scholar]
- 3.Hardie G, Hanks S. The Protein Kinase Facts Book. London: Academic; 1995. [Google Scholar]
- 4.Stock J B, Ninfa A J, Stock A M. Microbiol Rev. 1989;53:450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parkinson J S, Kofoid E C. Annu Rev Genet. 1992;26:71–112. doi: 10.1146/annurev.ge.26.120192.000443. [DOI] [PubMed] [Google Scholar]
- 6.Swanson R V, Alex L A, Simon M I. Trends Biochem Sci. 1994;19:485–490. doi: 10.1016/0968-0004(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 7.Chang C, Kwok S F, Bleecker A B, Meyerowitz E M. Science. 1993;262:539–544. doi: 10.1126/science.8211181. [DOI] [PubMed] [Google Scholar]
- 8.Ota I, Varshavsky A. Science. 1993;262:566–569. doi: 10.1126/science.8211183. [DOI] [PubMed] [Google Scholar]
- 9.Maeda T, Wurgler-Murphy S M, Saito H. Nature (London) 1994;369:242–245. doi: 10.1038/369242a0. [DOI] [PubMed] [Google Scholar]
- 10.Popov K M, Zhao Y, Shimamura Y, Kuntz M J, Harris R A. J Biol Chem. 1992;267:13127–13130. [PubMed] [Google Scholar]
- 11.Popov K M, Kedishvili N Y, Zhao Y, Shimamura Y, Crabb D W, Harris R A. J Biol Chem. 1993;268:26602–22606. [PubMed] [Google Scholar]
- 12.Maru Y, Witte O N. Cell. 1991;67:459–468. doi: 10.1016/0092-8674(91)90521-y. [DOI] [PubMed] [Google Scholar]
- 13.Beeler J F, La Rochelle W J, Chedid M, Tronick S R, Aaronson S A. Mol Cell Biol. 1994;14:982–988. doi: 10.1128/mcb.14.2.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dikstein R, Ruppert S, Tjian R. Cell. 1996;84:781–790. doi: 10.1016/s0092-8674(00)81055-7. [DOI] [PubMed] [Google Scholar]
- 15.Futey L M, Medley Q G, Cote G P, Egelhoff T T. J Biol Chem. 1995;270:523–529. doi: 10.1074/jbc.270.2.523. [DOI] [PubMed] [Google Scholar]
- 16.Eichinger L, Bomblies L, Vandekerckhove J, Schleicher M, Gettemans J. EMBO J. 1996;15:5547–5556. [PMC free article] [PubMed] [Google Scholar]
- 17.Ryazanov A G, Spirin A S. In: Translational Regulation of Gene Expression. Ilan J, editor. Vol. 2. New York: Plenum; 1993. pp. 433–455. [Google Scholar]
- 18.Nairn A C, Palfrey H C. In: Translational Control. Hershey J W B, Mathews M B, Sonenberg N, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1996. pp. 295–318. [Google Scholar]
- 19.Abdelmajid H, Leclerc-David C, Moreau M, Guerrier P, Ryazanov A G. Int J Dev Biol. 1993;37:279–290. [PubMed] [Google Scholar]
- 20.Ryazanov A G, Shestakova E A, Natapov P G. Nature (London) 1988;334:170–173. doi: 10.1038/334170a0. [DOI] [PubMed] [Google Scholar]
- 21.Palfrey H C, Nairn A C, Muldoon L L, Villereal M L. J Biol Chem. 1987;262:9785–9792. [PubMed] [Google Scholar]
- 22.Celis J E, Madsen P, Ryazanov A G. Proc Natl Acad Sci USA. 1990;87:4231–4235. doi: 10.1073/pnas.87.11.4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.End D, Hanson M, Hashimoto S, Guroff G. J Biol Chem. 1982;257:9223–9225. [PubMed] [Google Scholar]
- 24.Koizumi S, Ryazanov A G, Hama T, Chen H C, Guroff G. FEBS Lett. 1989;253:55–58. doi: 10.1016/0014-5793(89)80928-7. [DOI] [PubMed] [Google Scholar]
- 25.Brady M J, Nairn A C, Wagner J A, Palfrey H C. J Neurochem. 1990;54:1034–1039. doi: 10.1111/j.1471-4159.1990.tb02354.x. [DOI] [PubMed] [Google Scholar]
- 26.Severinov K V, Melnikova E G, Ryazanov A G. New Biol. 1990;2:887–893. [PubMed] [Google Scholar]
- 27.Bagaglio D M, Cheng E H, Gorelick F S, Mitsui K, Nairn A C, Hait W N. Cancer Res. 1993;53:2260–2264. [PubMed] [Google Scholar]
- 28.Hait W N, Ward M D, Trakht I N, Ryazanov A G. FEBS Lett. 1996;397:55–60. doi: 10.1016/s0014-5793(96)01140-4. [DOI] [PubMed] [Google Scholar]
- 29.Erdjument-Bromage H, Lui M, Sabatini D M, Snyder S H, Tempst P. Protein Sci. 1994;3:2435–2446. doi: 10.1002/pro.5560031227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elicone C, Lui M, Geromanos S, Erdjument-Bromage H, Tempst P. J Chromatogr. 1994;676:121–137. doi: 10.1016/0021-9673(94)00089-1. [DOI] [PubMed] [Google Scholar]
- 31.Feinberg A P, Vogelstein B. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- 32.Brenner S. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 34.Wilson R, Ainscough R, Anderson K, Baynes C, Berks M, et al. Nature (London) 1994;368:32–38. doi: 10.1038/368032a0. [DOI] [PubMed] [Google Scholar]
- 35.Redpath N T, Price N T, Proud C G. J Biol Chem. 1996;271:17547–17554. [PubMed] [Google Scholar]
- 36.Ptitsyn O B, Finkelstein A V. Biopolymers. 1983;22:15–25. doi: 10.1002/bip.360220105. [DOI] [PubMed] [Google Scholar]
- 37.Lupas A. Trends Biochem Sci. 1996;21:375–382. [PubMed] [Google Scholar]
- 38.Cote G P, Luo X, Murphy M B, Egelhoff T T. J Biol Chem. 1997;272:6846–6849. doi: 10.1074/jbc.272.11.6846. [DOI] [PubMed] [Google Scholar]
- 39.Taylor S S, Knighton D R, Zheng J, Ten Eyck L F, Sowadski J M. Annu Rev Cell Biol. 1992;8:429–462. doi: 10.1146/annurev.cb.08.110192.002241. [DOI] [PubMed] [Google Scholar]
- 40.Johnson L N, Noble M E M, Owen D J. Cell. 1996;85:149–158. doi: 10.1016/s0092-8674(00)81092-2. [DOI] [PubMed] [Google Scholar]
- 41.Donovan M G, Bodley J W. FEBS Lett. 1991;291:303–306. doi: 10.1016/0014-5793(91)81307-t. [DOI] [PubMed] [Google Scholar]