Abstract
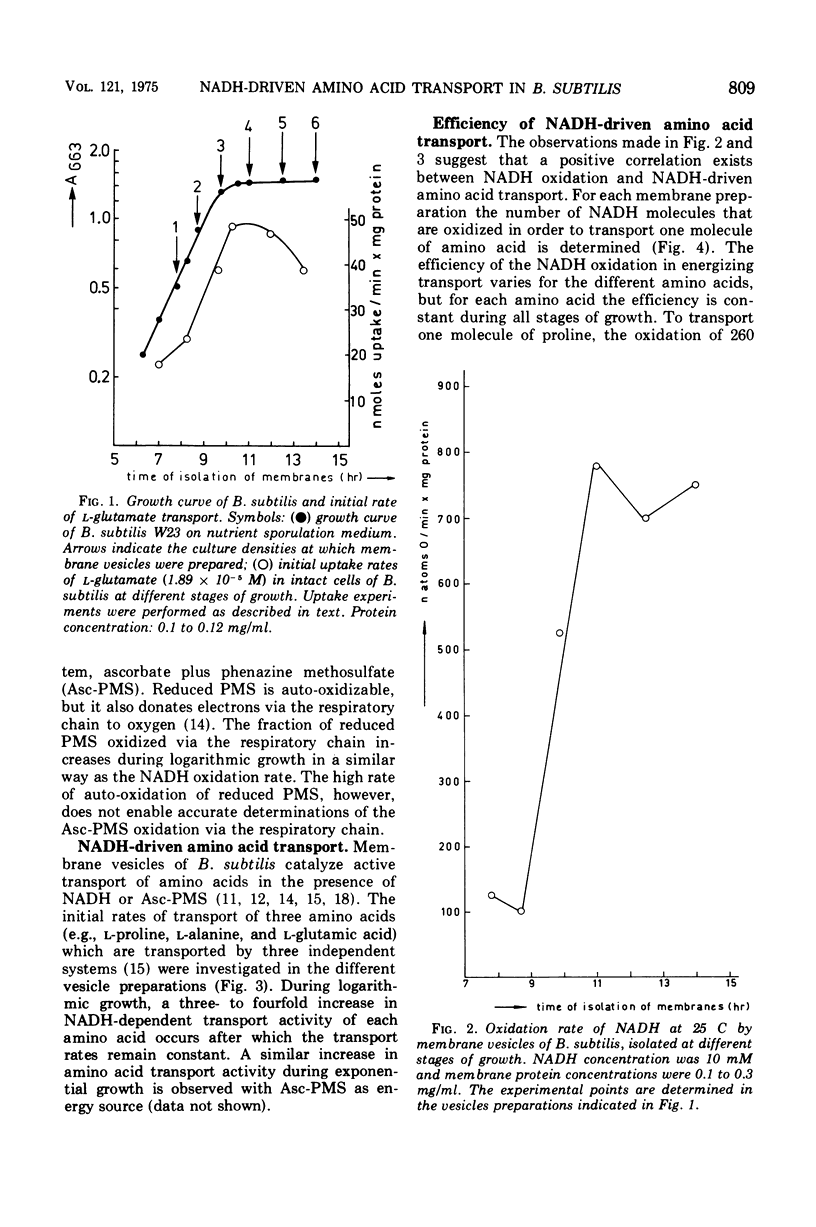
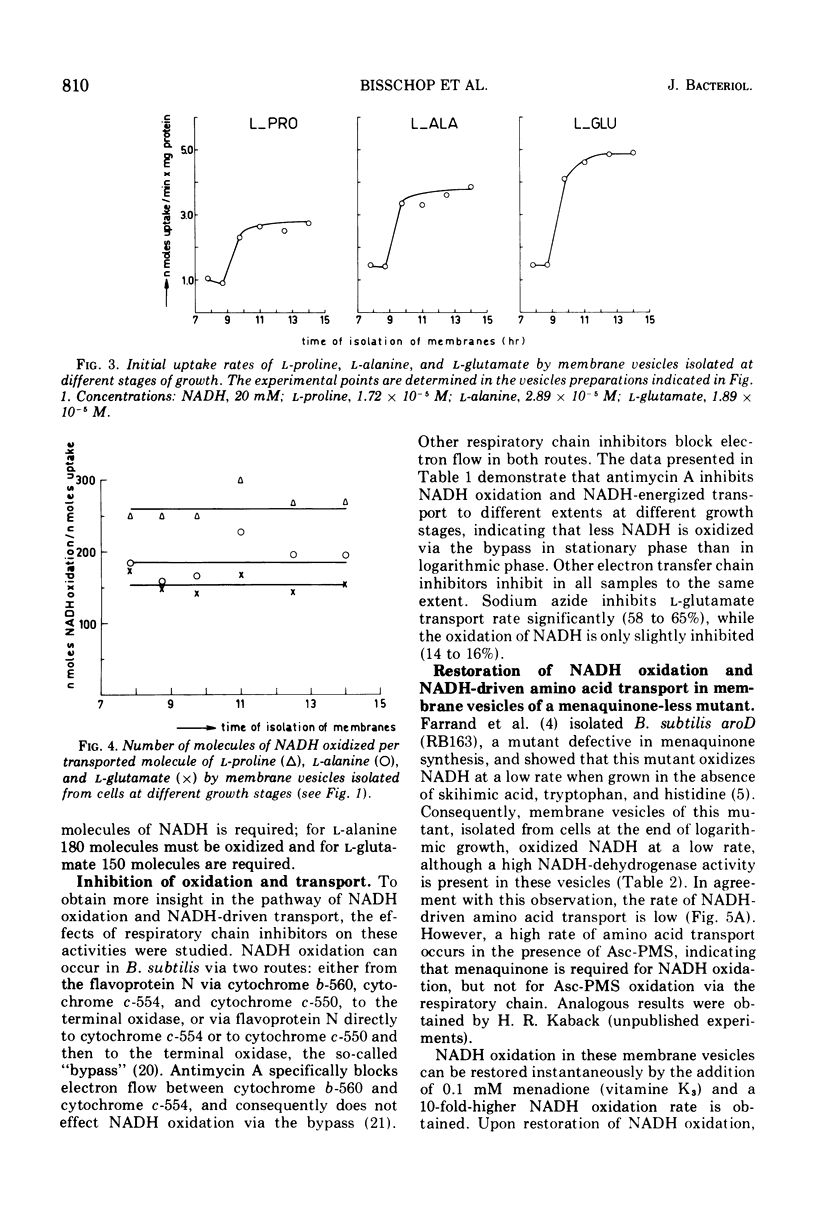
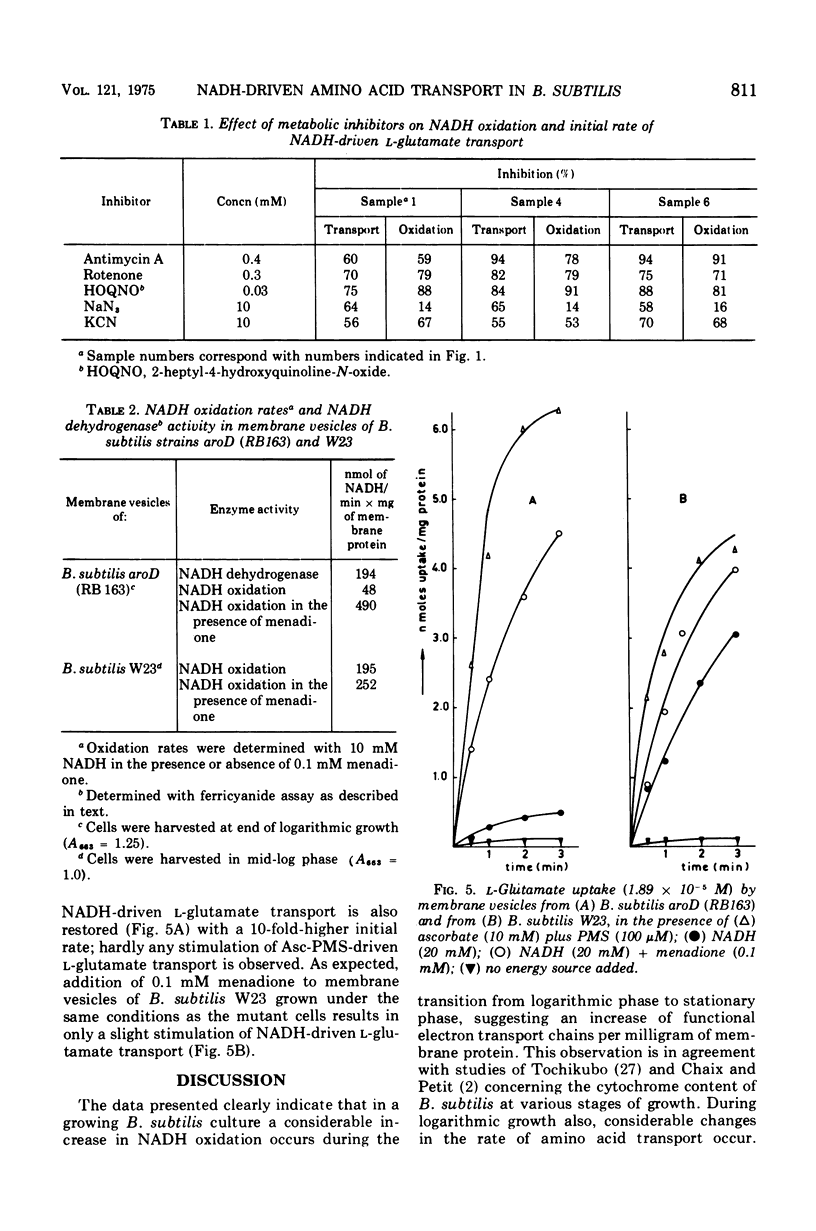
The rate of reduced nicotinamide adenine dinucleotide (NADH) oxidation by membrane vesicles from Bacillus subtilis W23 increases three- to fourfold during logarithmic growth, reaching maximal levels in early stationary phase. Initial rates of L-proline, L-alanine, and L-glutamate transport energized by NADH closely parallel the increase in NADH oxidation. In vesicles prepared at different stages of growth, a constant number of NADH molecules varying from 150 to 260 have to be oxidized to transport one molecule of amino acid. Membrane vesicles from B. subtilis aroD (strain RB163), a mutant defective in menaquinone synthesis, do not transport amino acids in the presence of NADH. Ascorbate plus phenazine methosulfate, however, energizes amino acid transport equally well as in vesicles of B. subtilis W23. NADH oxidation and NADH-driven amino acid transport can be restored instantaneously by the addition of menadione (vitamin K3).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes E. M., Jr, Kaback H. R. Beta-galactoside transport in bacterial membrane preparations: energy coupling via membrane-bounded D-lactic dehydrogenase. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1190–1198. doi: 10.1073/pnas.66.4.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAIX P., PETIT J. F. Etude de différents spectres cytochromiques de Bacillus subtilis. Biochim Biophys Acta. 1956 Oct;22(1):66–71. doi: 10.1016/0006-3002(56)90224-4. [DOI] [PubMed] [Google Scholar]
- Enoch H. G., Lester R. L. Effects of molybdate, tungstate, and selenium compounds on formate dehydrogenase and other enzyme systems in Escherichia coli. J Bacteriol. 1972 Jun;110(3):1032–1040. doi: 10.1128/jb.110.3.1032-1040.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrand S. K., Taber H. W. Changes in menaquinone concentration during growth and early sporulation in Bacillus subtilis. J Bacteriol. 1974 Jan;117(1):324–326. doi: 10.1128/jb.117.1.324-326.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrand S. K., Taber H. W. Pleiotropic menaquinone-deficient mutant of Bacillus subtilis. J Bacteriol. 1973 Sep;115(3):1021–1034. doi: 10.1128/jb.115.3.1021-1034.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortnagel P., Freese E. Analysis of sporulation mutants. II. Mutants blocked in the citric acid cycle. J Bacteriol. 1968 Apr;95(4):1431–1438. doi: 10.1128/jb.95.4.1431-1438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton M. L., Freese E. Explanation for the apparent inefficiency of reduced nicotinamide adenine dinucleotide in energizing amino acid transport in membrane vesicles. J Bacteriol. 1974 May;118(2):497–504. doi: 10.1128/jb.118.2.497-504.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R., Stadtman E. R. Glycine uptake in Escherichia coli. II. Glycine uptake, exchange, and metabolism by an isolated membrane preparation. J Biol Chem. 1968 Apr 10;243(7):1390–1400. [PubMed] [Google Scholar]
- Konings W. N., Barnes E. M., Jr, Kaback H. R. Mechanisms of active transport in isolated membrane vesicles. 2. The coupling of reduced phenazine methosulfate to the concentrative uptake of beta-galactosides and amino acids. J Biol Chem. 1971 Oct 10;246(19):5857–5861. [PubMed] [Google Scholar]
- Konings W. N., Bisschop A., Daatselaar M. C.C. Transport of L-glutamate and L-aspartate by membrane vesicles of Bacillus subtilis W 23. FEBS Lett. 1972 Aug 15;24(3):260–264. doi: 10.1016/0014-5793(72)80368-5. [DOI] [PubMed] [Google Scholar]
- Konings W. N., Bisschop A., Veenhuis M., Vermeulen C. A. New procedure for the isolation of membrane vesicles of Bacillus subtilis and an electron microscopy study of their ultrastructure. J Bacteriol. 1973 Dec;116(3):1456–1465. doi: 10.1128/jb.116.3.1456-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings W. N., Freese E. Amino acid transport in membrane vesicles of Bacillus subtilis. J Biol Chem. 1972 Apr 25;247(8):2408–2418. [PubMed] [Google Scholar]
- Konings W. N., Freese E. L-serine transport in membrane vesicles of Bacillus subtilis energized by NADH or reduced phenazine methosulfate. FEBS Lett. 1971 Apr 12;14(1):65–68. doi: 10.1016/0014-5793(71)80276-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MacLeod R. A., Thurman P., Rogers H. J. Comparative transport activity of intact cells, membrane vesicles, and mesosomes of Bacillus licheniformis. J Bacteriol. 1973 Jan;113(1):329–340. doi: 10.1128/jb.113.1.329-340.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A., Konings W. N., Kuenen J. G., Emmens M. Active transport of amino acids by membrane vesicles of Thiobacillus neapolitanus. J Gen Microbiol. 1974 Aug;83(2):311–318. doi: 10.1099/00221287-83-2-311. [DOI] [PubMed] [Google Scholar]
- Matin A., Konings W. N. Transport of lactate and succinate by membrane vesicles of Escherichia coli, Bacillus subtilis and a pseudomonas species. Eur J Biochem. 1973 Apr 2;34(1):58–67. doi: 10.1111/j.1432-1033.1973.tb02728.x. [DOI] [PubMed] [Google Scholar]
- Miki K., Okunuki K. Cytochromes of Bacillus subtilis. 3. Physicochemical and enzymatic properties of cytochromes c-550 and c-554. J Biochem. 1969 Dec;66(6):845–854. doi: 10.1093/oxfordjournals.jbchem.a129215. [DOI] [PubMed] [Google Scholar]
- Milner L. S., Kaback H. R. The role of phosphatidylglycerol in the vectorial phosphorylation of sugar by isolated bacterial membrane preparations. Proc Natl Acad Sci U S A. 1970 Mar;65(3):683–690. doi: 10.1073/pnas.65.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Short S. A., Kaback H. R., Kohn L. D. D-lactate dehydrogenase binding in Escherichia coli dld- membrane vesicles reconstituted for active transport. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1461–1465. doi: 10.1073/pnas.71.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short S. A., White D. C., Kaback H. R. Active transport in isolated bacterial membrane vesicles. V. The transport of amino acids by membrane vesicles prepared from Staphylococcus aureus. J Biol Chem. 1972 Jan 10;247(1):298–304. [PubMed] [Google Scholar]
- Thomas E. L., Weissbach H., Kaback H. R. Further studies on metabolism of phosphatidic acid of isolated E. coli membrane vesicles. Arch Biochem Biophys. 1972 Jun;150(2):797–806. doi: 10.1016/0003-9861(72)90101-4. [DOI] [PubMed] [Google Scholar]
- Tochikubo K. Changes in terminal respiratory pathways of Bacillus subtilis during germination, outgrowth and vegetative growth. J Bacteriol. 1971 Nov;108(2):652–661. doi: 10.1128/jb.108.2.652-661.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


