Abstract
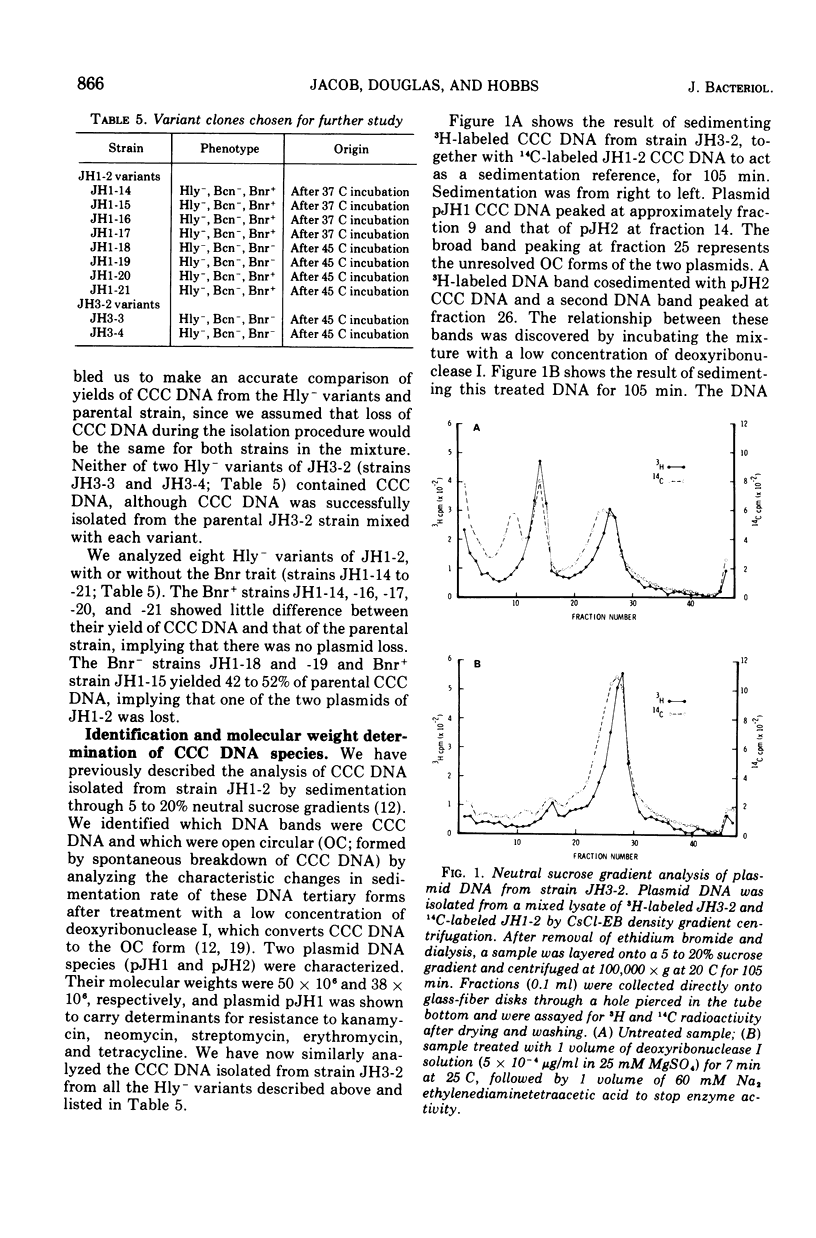
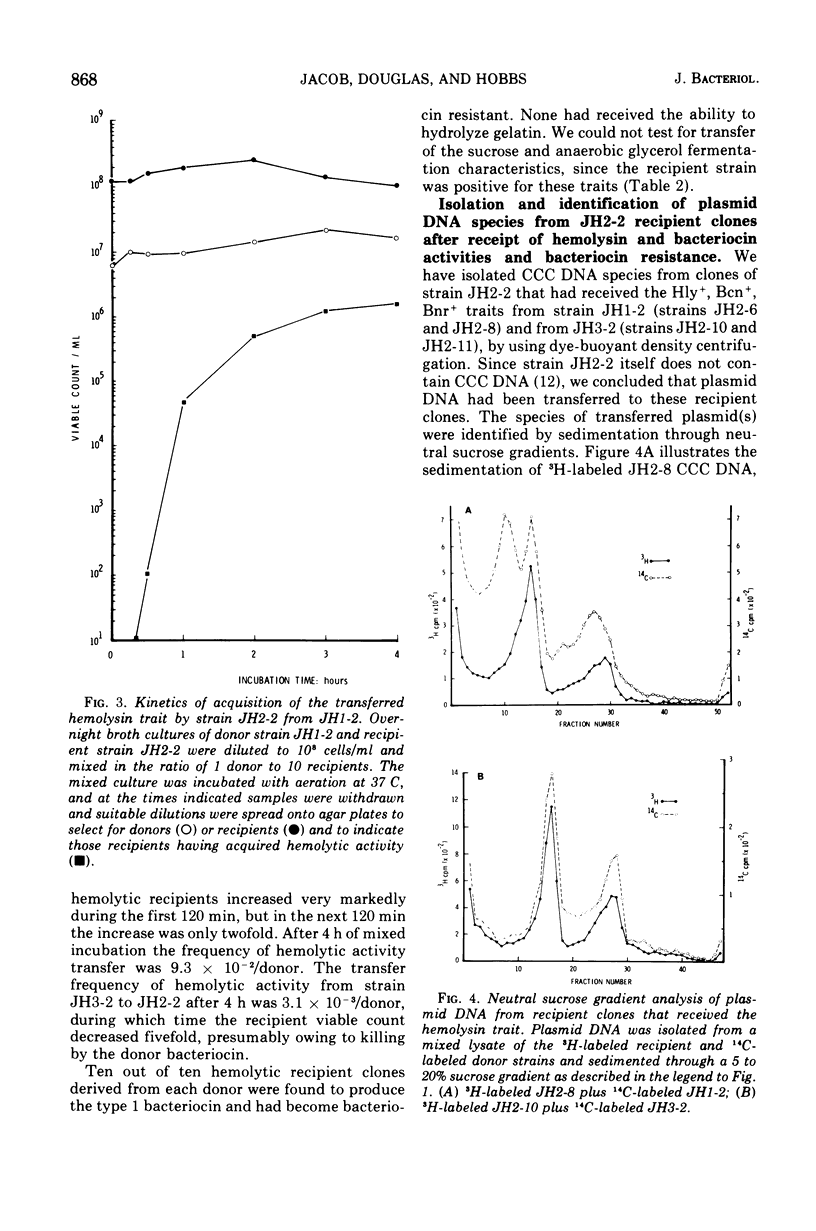
Strains of Streptococcus faecalis var. zymogenes, designated JH1 and JH3, produced a hemolysin and a bacteriocin. Hemolytic activity was lost from a low percentage of cells grown in broth at either 37 or 45 C. All nonhemolytic (Hly-) variants had lost bacteriocin activity (Ben-), and those from strain JH3 had also lost resistance to the bacteriocin (Bnr-). The majority of Hly-, Ben- variants from JH1 retained bacteriocin resistance (Bnrplus). Strains JH1 and JH3 contained a plasmid deoxyribonucleic acid species of molecular weight 38 times 10-6 (plasmids pJH2 and pJH3, respectively), and strain JH1 also contained a 50 times 10-6 molecular weight plasmid (pJH1) which has previously been shown to carry the genes determining resistance to the antibiotics kanamycin, neomycin, streptomycin, erythromycin, and tetracycline. Hly-, Bcn-, Bnr- variants of strain JH3 had completely lost plasmid pJH3. Hly-, Bcn-, Bnr- variants of strain JH1 had completely lost plasmid pJH2 and retained plasmid pJH1, but Hly-, Bcn-, Bnrplus variants had retained both plasmids pJH2 and pJH1. The Hlyplus, Bcnplus, Bnrplus traits from both parental strains were transferable to nonhemolytic S. faecalis strains during mixed incubation in broth at 37 C, and hemolytic recipient strains were found to have received plasmid pJH2 from strain JH1 and pJH3 from JH3. We conclude that the Hlyplus, Bnrplus traits are borne on plasmid pJH2 in strain JH1 and pJH3 in strain JH3 and that, in Hly-, Bcn-, Bnrplus variants of strain JH1, plasmic pJH2 has suffered a mutation affecting hemolysin and bacteriocin expression. We infer that the plasmids transfer by conjugation. Beta-hemolytic activity is the only property distinguishing the zymogenes variety from S. faecalis. Since we have shown that this activity is plasmid borne in strains JH1 and JH3, we endorse the view that the varietal status of zymogenes should be dropped.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROCK T. D., DAVIE J. M. PROBABLE IDENTITY OF A GROUP D HEMOLYSIN WITH A BACTERIOCINE. J Bacteriol. 1963 Oct;86:708–712. doi: 10.1128/jb.86.4.708-712.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROCK T. D. HOST RANGE OF CERTAIN VIRULENT AND TEMPERATE BACTERIOPHAGES ATTACKING GROUP D STREPTOCOCCI. J Bacteriol. 1964 Jul;88:165–171. doi: 10.1128/jb.88.1.165-171.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROCK T. D., PEACHER B., PIERSON D. SURVEY OF THE BACTERIOCINES OF ENTEROCOCCI. J Bacteriol. 1963 Oct;86:702–707. doi: 10.1128/jb.86.4.702-707.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak A. L., Christiansen C., Stenderup A. Bacterial genome sizes determined by DNA renaturation studies. J Gen Microbiol. 1970 Dec;64(3):377–380. doi: 10.1099/00221287-64-3-377. [DOI] [PubMed] [Google Scholar]
- Clowes R. C. Molecular structure of bacterial plasmids. Bacteriol Rev. 1972 Sep;36(3):361–405. doi: 10.1128/br.36.3.361-405.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEIBEL R. H. HYDROLYSIS OF PROTEINS AND NUCLEIC ACIDS BY LANCEFIELD GROUP A AND OTHER STREPTOCOCCI. J Bacteriol. 1963 Dec;86:1270–1274. doi: 10.1128/jb.86.6.1270-1274.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEIBEL R. H., LAKE D. E., NIVEN C. F., Jr PHYSIOLOGY OF THE ENTEROCOCCI AS RELATED TO THEIR TAXONOMY. J Bacteriol. 1963 Dec;86:1275–1282. doi: 10.1128/jb.86.6.1275-1282.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEIBEL R. H. THE GROUP D STREPTOCOCCI. Bacteriol Rev. 1964 Sep;28:330–366. doi: 10.1128/br.28.3.330-366.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granato P. A., Jackson R. W. Bicomponent nature of lysin from Streptococcus zymogenes. J Bacteriol. 1969 Nov;100(2):865–868. doi: 10.1128/jb.100.2.865-868.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granato P. A., Jackson R. W. Characterization of the A component of Streptococcus zymogenes lysin. J Bacteriol. 1971 Aug;107(2):551–556. doi: 10.1128/jb.107.2.551-556.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granato P. A., Jackson R. W. Purification and characterization of the L component of Streptococcus zymogenes lysin. J Bacteriol. 1971 Nov;108(2):804–808. doi: 10.1128/jb.108.2.804-808.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A. E., Hobbs S. J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974 Feb;117(2):360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P. Extrachromosomal inheritance in bacteria. Bacteriol Rev. 1969 Jun;33(2):210–263. doi: 10.1128/br.33.2.210-263.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAYCROFT R. E., ZIMMERMAN L. N. NEW MODE OF GENETIC TRANSFER IN STREPTOCOCCUS FAECALIS VAR. LIQUEFACIENS. J Bacteriol. 1964 Apr;87:799–801. doi: 10.1128/jb.87.4.799-801.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Sherman J. M., Stark P., Mauer J. C. Streptococcus zymogenes. J Bacteriol. 1937 May;33(5):483–494. doi: 10.1128/jb.33.5.483-494.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman J. M. The Enterococci and Related Streptococci. J Bacteriol. 1938 Feb;35(2):81–93. doi: 10.1128/jb.35.2.81-93.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomura T., Hirano T., Ito T., Yoshioka M. Transmission of bacteriocinogenicity by conjugation in group D streptococci. Jpn J Microbiol. 1973 Nov;17(6):445–452. doi: 10.1111/j.1348-0421.1973.tb00930.x. [DOI] [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J., Radloff R., Watson R., Laipis P. The twisted circular form of polyoma viral DNA. Proc Natl Acad Sci U S A. 1965 May;53(5):1104–1111. doi: 10.1073/pnas.53.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]



