Abstract
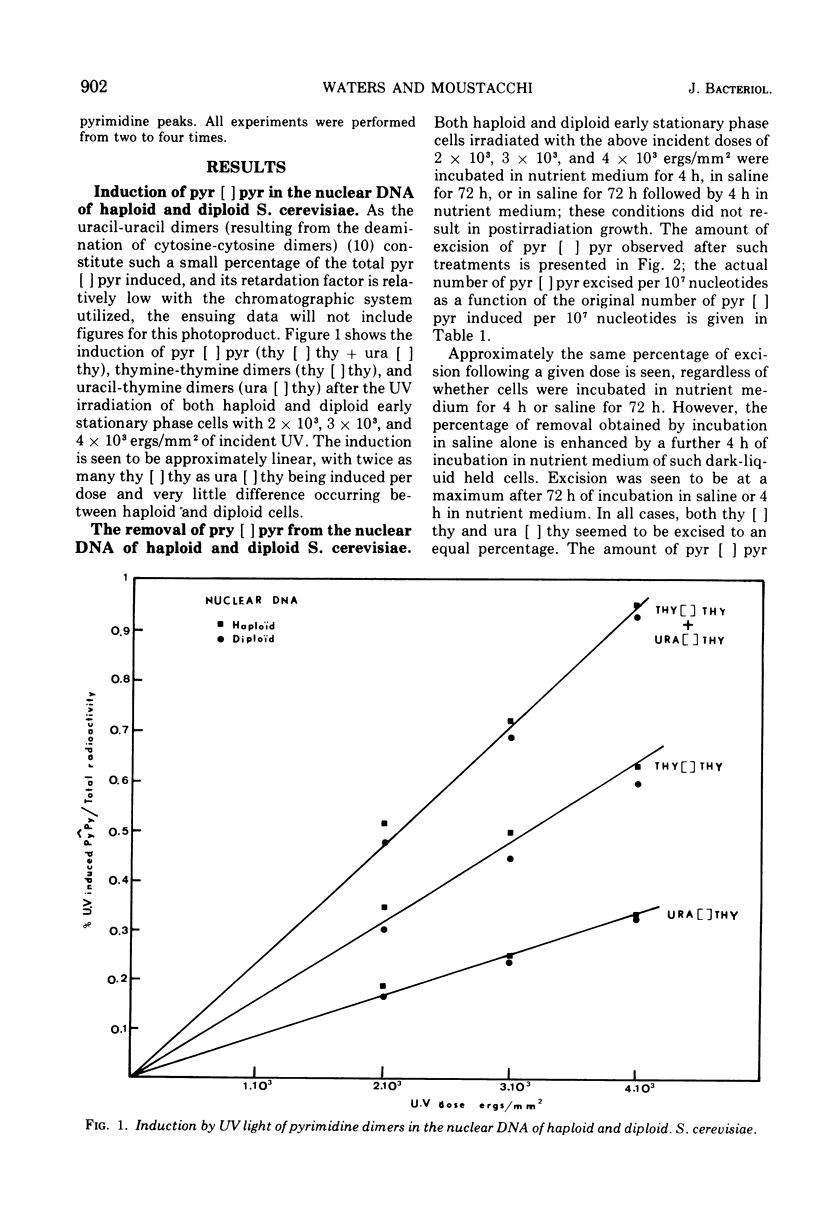
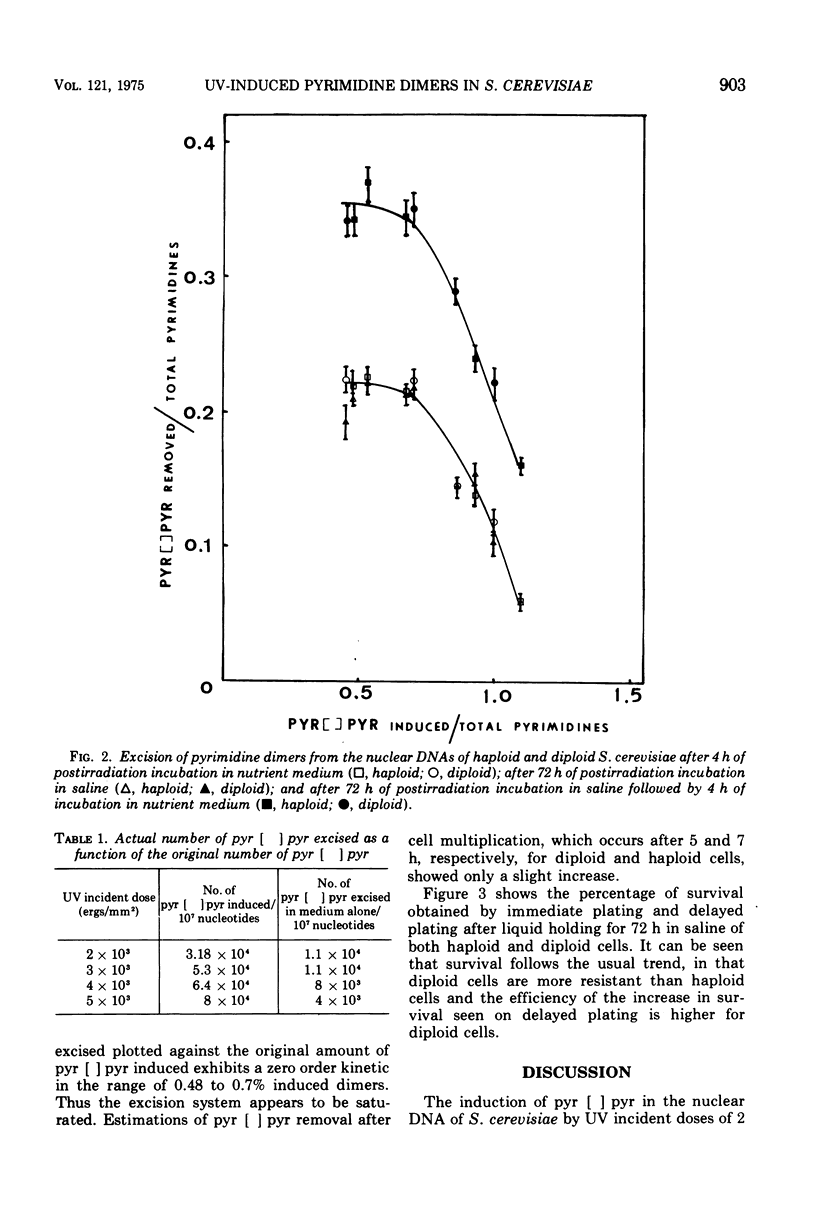
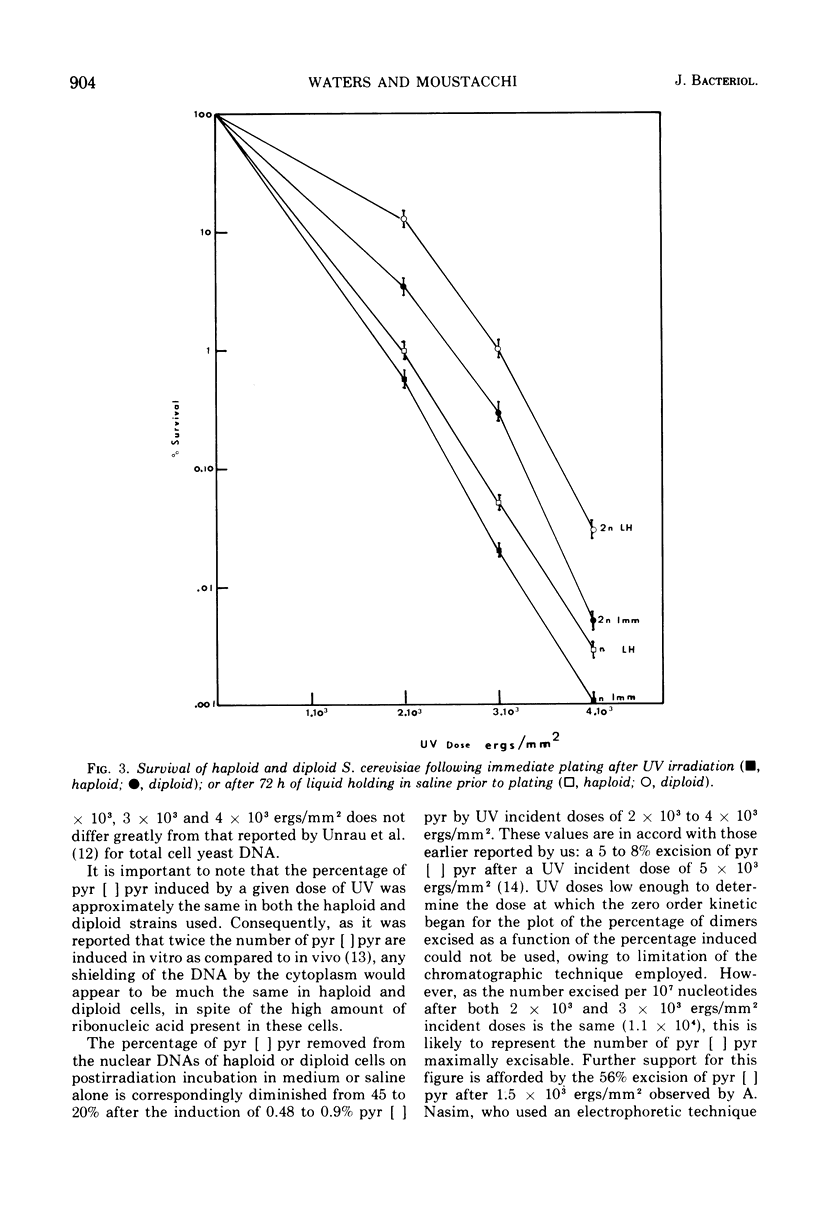
The yield of ultraviolet-induced dimers is similar for a fixed dose in both haploid and diploid Saccharomyces cerevisiae. The excision of these photo-products from the nuclear deoxyribonucleic acids of cells of both ploidies after ultraviolet incident doses of 2 times 10-3 to 4 times 10-3 ergs/mm2 decreased with the corresponding increasing dose. Postirradiation incubation in saline followed by a further incubation in nutrient medium increases the excision as compared to that seen in either nutrient medium or saline alone. Previous data regarding both pyrimidine dimer removal and the survival of haploid and diploid cells after ultraviolet irradiation and either immediate or delayed plating are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyle J. M., Setlow R. B. Correlations between host-cell reactivation, ultraviolet reactivation and pyrimidine dimer excision in the DNA of bacteriophage lambda. J Mol Biol. 1970 Jul 14;51(1):131–144. doi: 10.1016/0022-2836(70)90275-5. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. DNA repair and radiation sensitivity in human (xeroderma pigmentosum) cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;18(6):557–565. doi: 10.1080/09553007014551491. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E., Trosko J. E. Absence of excision of ultraviolet-induced cyclobutane dimers in xeroderma pigmentosum. Photochem Photobiol. 1970 Jun;11(6):547–550. doi: 10.1111/j.1751-1097.1970.tb06025.x. [DOI] [PubMed] [Google Scholar]
- Cohn W. E., Leonard N. J., Wang S. Y. Abbreviations for pyrimidine photoproducts. Photochem Photobiol. 1974 Feb;19(2):89–94. doi: 10.1111/j.1751-1097.1974.tb06482.x. [DOI] [PubMed] [Google Scholar]
- Moustacchi E., Enteric S. Differential "liquid holding recovery" for the lethal effect and cytoplasmic "petite" induction by UV light in Saccharomyces cerevisiae. Mol Gen Genet. 1970;109(1):69–83. doi: 10.1007/BF00334047. [DOI] [PubMed] [Google Scholar]
- PATRICK M. H., HAYNES R. H., URETZ R. B. DARK RECOVERY PHENOMENA IN YEAST. 1. COMPARATIVE EFFECTS WITH VARIOUS INACTIVATING AGENTS. Radiat Res. 1964 Jan;21:144–163. [PubMed] [Google Scholar]
- Paterson M. C., Lohman P. H., Sluyter M. L. Use of UV endonuclease from Micrococcus luteus to monitor the progress of DNA repair in UV-irradiated human cells. Mutat Res. 1973 Aug;19(2):245–256. doi: 10.1016/0027-5107(73)90083-3. [DOI] [PubMed] [Google Scholar]
- Resnick M. A., Setlow J. K. Repair of pyrimidine dimer damage induced in yeast by ultraviolet light. J Bacteriol. 1972 Mar;109(3):979–986. doi: 10.1128/jb.109.3.979-986.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow R. B., Carrier W. L. Pyrimidine dimers in ultraviolet-irradiated DNA's. J Mol Biol. 1966 May;17(1):237–254. doi: 10.1016/s0022-2836(66)80105-5. [DOI] [PubMed] [Google Scholar]
- Störl K., Mund C., Venner H. Repair defect mutants of Proteus mirabilis. II. Excision of pyrimidine dimers from the DNA of ultraviolet-irradiated P. mirabilis wildtype and UV-sensitive mutants. Mol Gen Genet. 1973 Aug 17;124(3):259–268. doi: 10.1007/BF00293097. [DOI] [PubMed] [Google Scholar]
- Unrau P., Wheatcroft R., Cox B. S. The excision of pyrimidine dimers from DNA of ultraviolet irradiated yeast. Mol Gen Genet. 1971;113(4):359–362. doi: 10.1007/BF00272336. [DOI] [PubMed] [Google Scholar]
- Unrau P., Wheatcroft R., Cox B., Olive T. The formation of pyrimidine dimers in the DNA of fungi and bacteria. Biochim Biophys Acta. 1973 Jul 27;312(4):626–632. doi: 10.1016/0005-2787(73)90065-8. [DOI] [PubMed] [Google Scholar]
- Waters R., Moustacchi E. The disappearance of ultraviolet-induced pyrimidine dimers from the nuclear DNA of exponential and stationary phase cells of Saccharomyces cerevisiae following various post-irradiation treatments. Biochim Biophys Acta. 1974 Jul 24;353(4):407–419. doi: 10.1016/0005-2787(74)90048-3. [DOI] [PubMed] [Google Scholar]
- Waters R., Moustacchi E. The fate of ultraviolet-induced pyrimidine dimers in the mitochondrial DNA of Saccharomyces cerevisiae following various post-irradiation cell treatments. Biochim Biophys Acta. 1974 Oct 28;366(3):241–250. doi: 10.1016/0005-2787(74)90282-2. [DOI] [PubMed] [Google Scholar]
- Waters R., Parry J. M. A comparative study of the effects of UV irradiation upon diploid cultures of yeast defective at the rad 3 locus. Mol Gen Genet. 1973 Aug 10;124(2):145–156. doi: 10.1007/BF00265147. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Moustacchi E., Fennell D. A procedure for rapidly extracting and estimating the nuclear and cytoplasmic DNA components of yeast cells. Biochim Biophys Acta. 1971 May 13;238(2):369–374. doi: 10.1016/0005-2787(71)90106-7. [DOI] [PubMed] [Google Scholar]


