Abstract
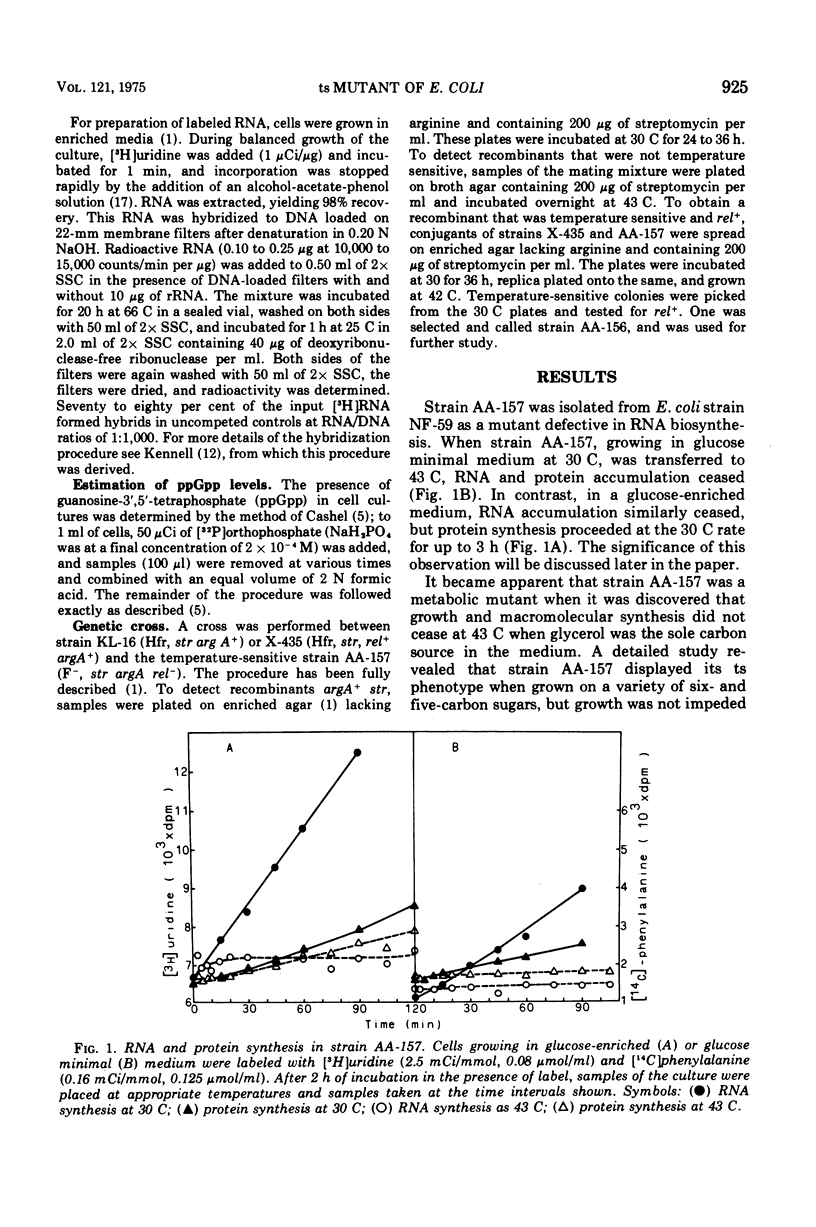
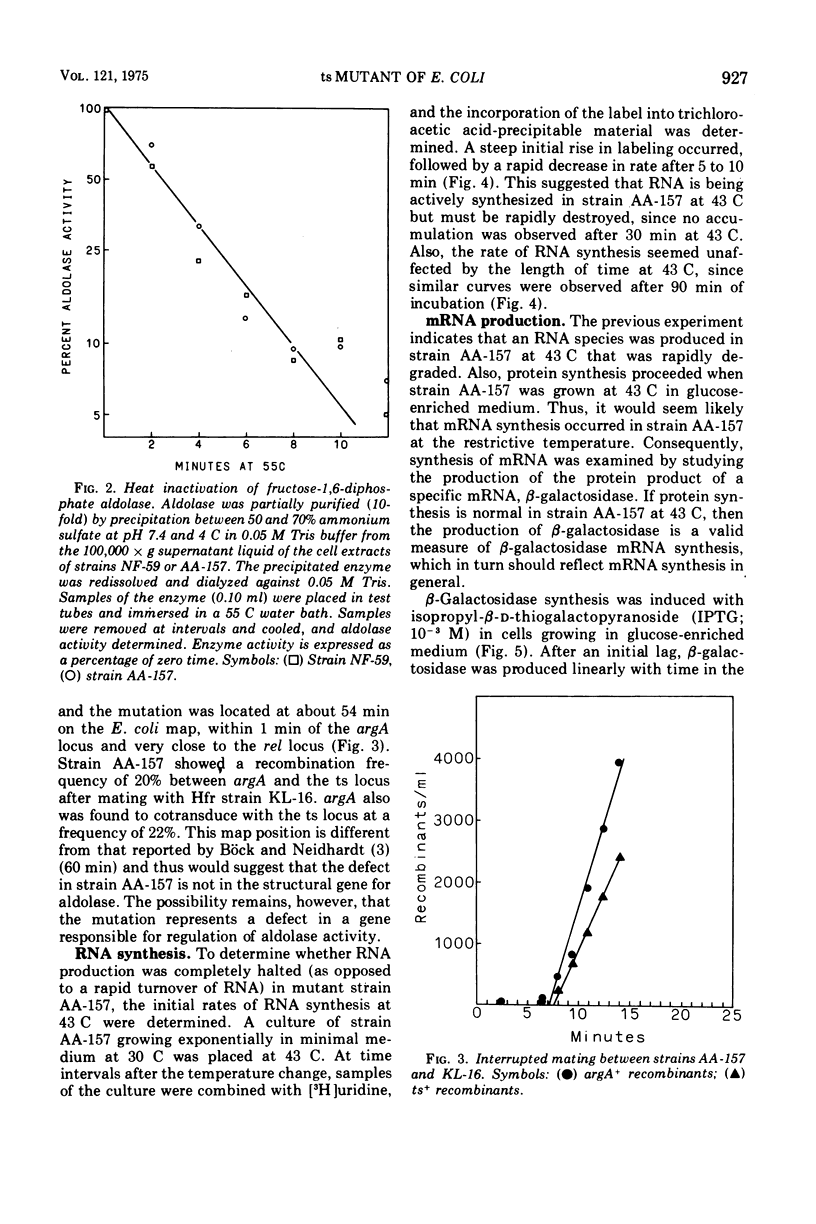
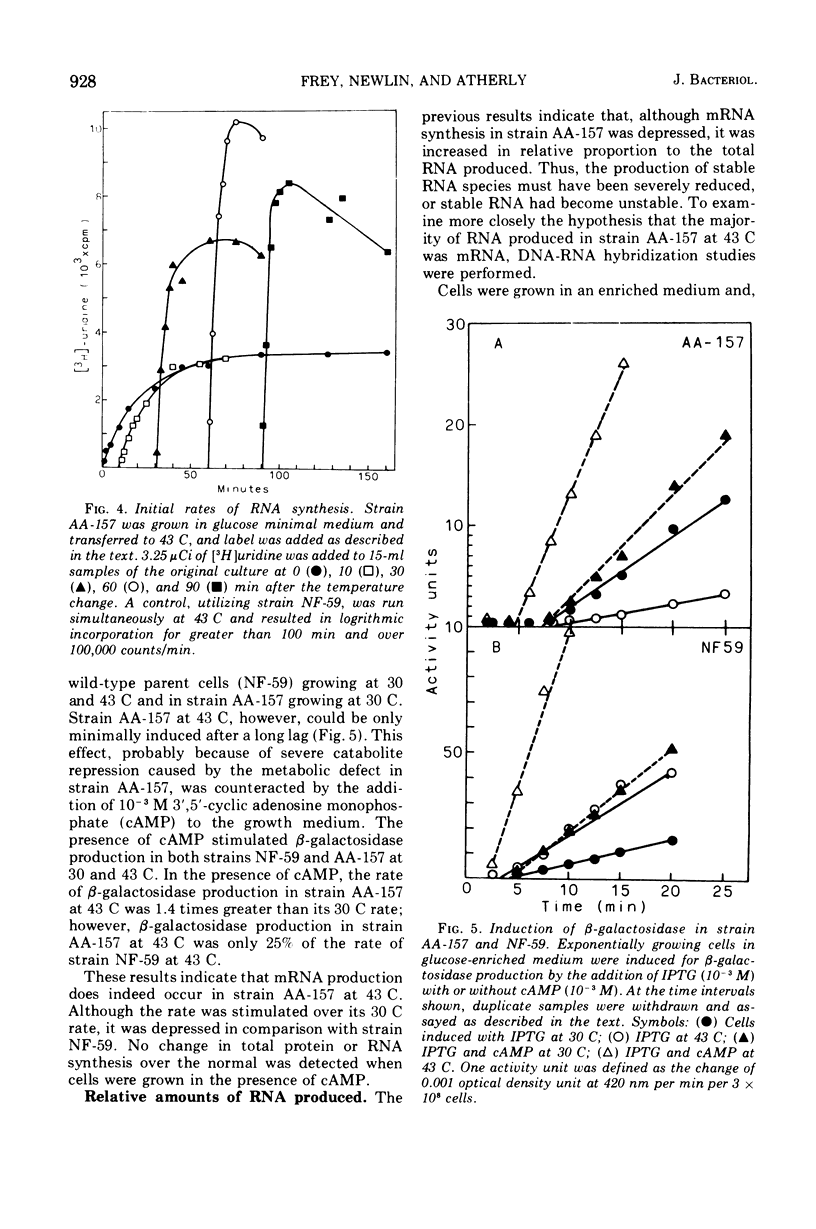
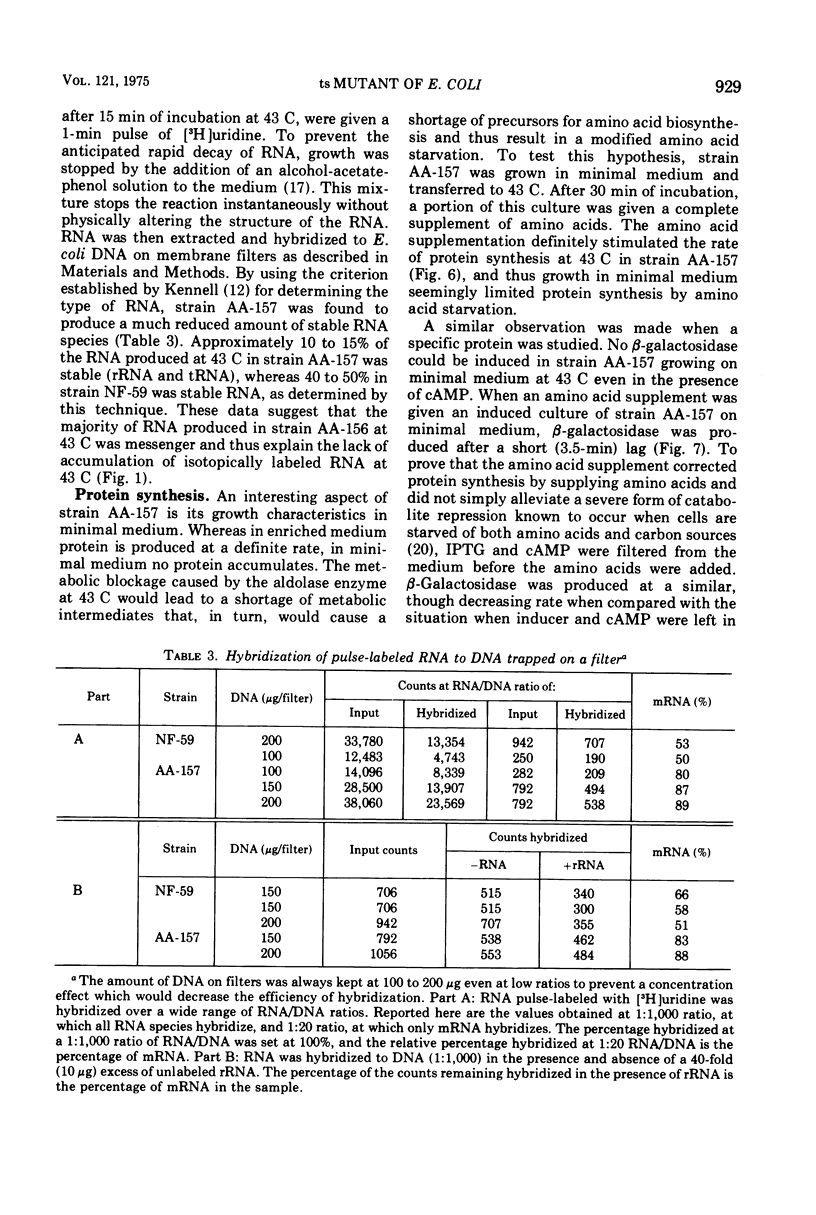
A mutant of Escherichia coli has been isolated that has a temperature-sensitive mutation that results in specific loss of ribosomal ribonucleic acid (RNA) synthesis and some reduction in messenger RNA synthesis. When the strain was grown in glucose medium at a restrictive temperature, RNA accumulation ceased, but both messenger RNA and protein synthesis continued for an extended time. Because carbon metabolism was slowed drastically when strain AA-157 was placed at the restrictive temperature, this phenotype can be compared with carbon depletion conditions present during diauxic lag. However, the phenotype of mutant AA-157 differs from shift-down conditions in that guanosine-3',5'-tetraphosphate levels are unaffected; therefore, a different site is affected. This mutant strain (AA-157) thus shows many characteristics similar to an aldolase mutant previously reported (Böck and Neidhardt, 1966). However, the mutation occurred in a different position on the E. coli genetic map, and furthermore, aldolase was not temperature sensitive in strain AA-157. In this paper we present a study of macromolecular biosynthesis in this mutant.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atherly A. G., Suchanek M. C. Characterization of mutants of Escherichia coli temperature-sensitive for ribonucleic acid regulation: an unusual phenotype associated with a phenylalanyl transfer ribonucleic acid synthetase mutant. J Bacteriol. 1971 Nov;108(2):627–638. doi: 10.1128/jb.108.2.627-638.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOREK E., RYAN A., ROCKENBACH J. Nucleic acid metabolism in relation to the lysogenic phenomenon. J Bacteriol. 1955 Apr;69(4):460–467. doi: 10.1128/jb.69.4.460-467.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böck A., Neidhardt F. C. Isolation of a Mutant of Escherichia coli with a Temperature-sensitive Fructose-1,6-Diphosphate Aldolase Activity. J Bacteriol. 1966 Aug;92(2):464–469. doi: 10.1128/jb.92.2.464-469.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böck A., Neidhardt F. C. Properties of a Mutant of Escherichia coli with a Temperature-sensitive Fructose-1,6-Diphosphate Aldolase. J Bacteriol. 1966 Aug;92(2):470–476. doi: 10.1128/jb.92.2.470-476.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashel M. The control of ribonucleic acid synthesis in Escherichia coli. IV. Relevance of unusual phosphorylated compounds from amino acid-starved stringent strains. J Biol Chem. 1969 Jun 25;244(12):3133–3141. [PubMed] [Google Scholar]
- EIDLIC L., NEIDHARDT F. C. PROTEIN AND NUCLEIC ACID SYNTHESIS IN TWO MUTANTS OF ESCHERICHIA COLI WITH TEMPERATURE-SENSITIVE AMINOACYL RIBONUCLEIC ACID SYNTHETASES. J Bacteriol. 1965 Mar;89:706–711. doi: 10.1128/jb.89.3.706-711.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fill N. A functional analysis of the rel gene in Escherichia coli. J Mol Biol. 1969 Oct 28;45(2):195–203. doi: 10.1016/0022-2836(69)90099-0. [DOI] [PubMed] [Google Scholar]
- Jacobson L. A. Control of stable ribonucleic acid chain initiation in Escherichia coli during diauxie lag. J Bacteriol. 1972 Feb;109(2):678–685. doi: 10.1128/jb.109.2.678-685.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L. A. Regulation of ribonucleic acid synthesis in Escherichia coli during diauxie lag: accumulation of heterogeneous ribonucleic acid. J Bacteriol. 1970 Jun;102(3):740–746. doi: 10.1128/jb.102.3.740-746.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURLAND C. G., MAALOE O. Regulation of ribosomal and transfer RNA synthesis. J Mol Biol. 1962 Mar;4:193–210. doi: 10.1016/s0022-2836(62)80051-5. [DOI] [PubMed] [Google Scholar]
- Kaplan S., Atherly A. G., Barrett A. Synthesis of stable RNA in stringent Escherichia coli cells in the absence of charged transfer RNA. Proc Natl Acad Sci U S A. 1973 Mar;70(3):689–692. doi: 10.1073/pnas.70.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennel D. Titration of the gene sites on DNA by DNA-RNA hybridization. II. The Escherichia coli chromosome. J Mol Biol. 1968 May 28;34(1):85–103. doi: 10.1016/0022-2836(68)90236-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lazzarini R. A., Dahlberg A. E. The control of ribonucleic acid synthesis during amino acid deprivation in Escherichia coli. J Biol Chem. 1971 Jan 25;246(2):420–429. [PubMed] [Google Scholar]
- Manor H., Goodman D., Stent G. S. RNA chain growth rates in Escherichia coli. J Mol Biol. 1969 Jan 14;39(1):1–29. doi: 10.1016/0022-2836(69)90329-5. [DOI] [PubMed] [Google Scholar]
- Morris D. W., Kjeldgaard N. O. Evidence for the non-co-ordinate regulation of ribonucleic acid synthesis in stringent strains of Escherichia coli. J Mol Biol. 1968 Jan 14;31(1):145–148. doi: 10.1016/0022-2836(68)90064-8. [DOI] [PubMed] [Google Scholar]
- NAKADA D., MAGASANIK B. THE ROLES OF INDUCER AND CATABOLITE REPRESSOR IN THE SYNTHESIS OF BETA-GALACTOSIDASE BY ESCHERICHIA COLI. J Mol Biol. 1964 Jan;8:105–127. doi: 10.1016/s0022-2836(64)80153-4. [DOI] [PubMed] [Google Scholar]
- NEIDHARDT F. C., MAGASANIK B. Studies on the role of ribonucleic acid in the growth of bacteria. Biochim Biophys Acta. 1960 Jul 29;42:99–116. doi: 10.1016/0006-3002(60)90757-5. [DOI] [PubMed] [Google Scholar]
- Norris T. E., Koch A. L. Effect of growth rate on the relative rates of synthesis of messenger, ribosomal and transfer RNA in Escherichia coli. J Mol Biol. 1972 Mar 14;64(3):633–649. doi: 10.1016/0022-2836(72)90088-5. [DOI] [PubMed] [Google Scholar]
- Rosset R., Julien J., Monier R. Ribonucleic acid composition of bacteria as a function of growth rate. J Mol Biol. 1966 Jul;18(2):308–320. doi: 10.1016/s0022-2836(66)80248-6. [DOI] [PubMed] [Google Scholar]
- SCHAECHTER M., MAALOE O., KJELDGAARD N. O. Dependency on medium and temperature of cell size and chemical composition during balanced grown of Salmonella typhimurium. J Gen Microbiol. 1958 Dec;19(3):592–606. doi: 10.1099/00221287-19-3-592. [DOI] [PubMed] [Google Scholar]
- STENT G. S., BRENNER S. A genetic locus for the regulation of ribonucleic acid synthesis. Proc Natl Acad Sci U S A. 1961 Dec 15;47:2005–2014. doi: 10.1073/pnas.47.12.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salser W., Gesteland R. F., Bolle A. In vitro synthesis of bacteriophage lysozyme. Nature. 1967 Aug 5;215(5101):588–591. doi: 10.1038/215588a0. [DOI] [PubMed] [Google Scholar]
- Sarkar S., Moldave K. Characterization of the ribonucleic acid synthesized during amino acid-deprivation of a stringent auxotroph of Escherichia coli. J Mol Biol. 1968 Apr 14;33(1):213–224. doi: 10.1016/0022-2836(68)90289-1. [DOI] [PubMed] [Google Scholar]
- Winslow R. M. A consequence of the rel gene during a glucose to lactate downshift in Escherichia coli. The rates of ribonucleic acid synthesis. J Biol Chem. 1971 Aug 10;246(15):4872–4877. [PubMed] [Google Scholar]


