Abstract
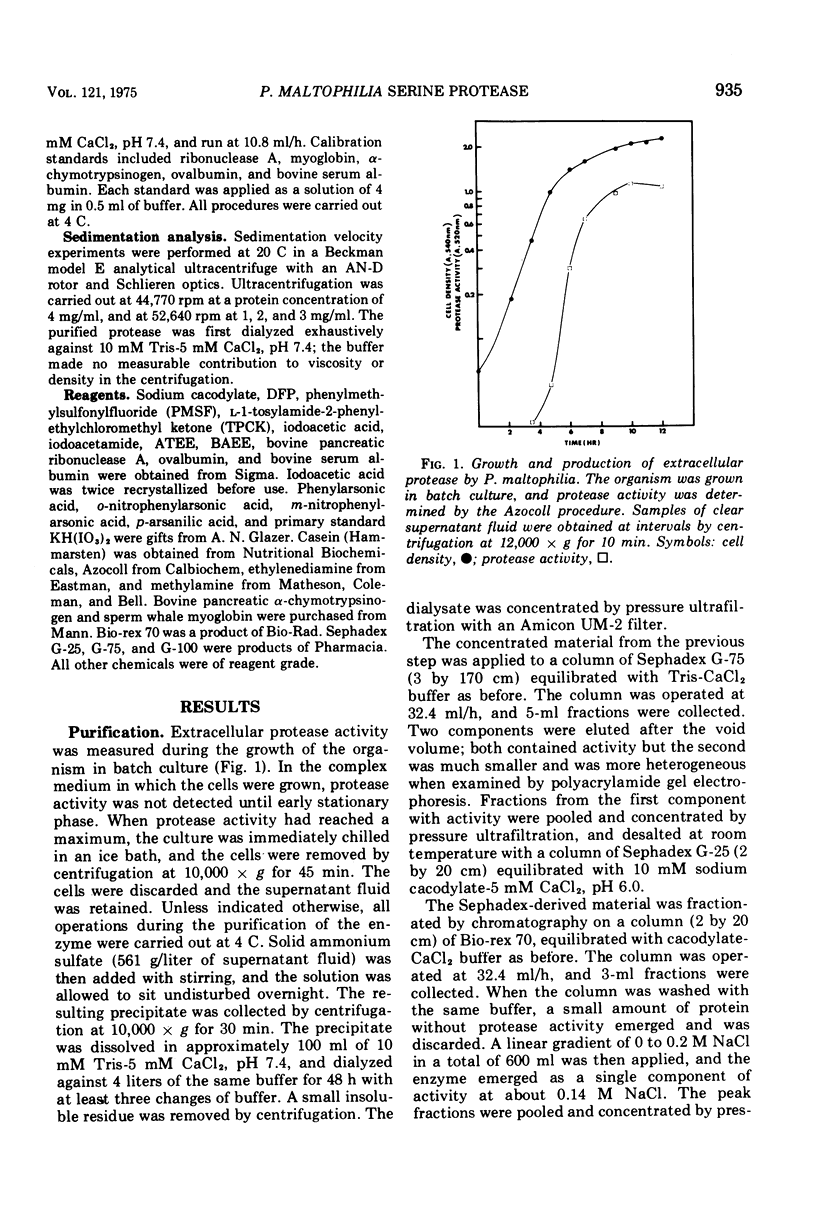
The extracellular protease of Pseudomonas maltophilia was partially purified by ammonium sulfate precipitation and chromatography on Sephadex G-75 and Bio-rex 70. Gel electrophoresis revealed minor impurities. The enzyme exhibited the following properties: (i) molecular weight, 35,000; (ii) A see article; 10.8; (iii) isoelectric point, 9.3; (iv) pH optimum, 10.0; (v)s20, w equal 3.47. The enzyme was rapidly inactivated by ethylenediaminetetracetate, but activity could be partially restored with divalent cations. Of those tested, Ca2+, Sr2+, Ba2+, Co2+, Cu2+, Mg2+, and Zn2+ were all effective. Both phenylmethylsulfonylfluoride and diisopropylfluorophosphate were powerful inhibitors of protease activity, but L-1-tosylamide-2-phenylethylchloromethyl ketone, iodoacetic acid, and iodoacetamide were without effect. The enzyme hydrolyzed the esters N-acetyl-L-tyrosine ethyl ester and alpha-N-benzoyl-L-arginine ethyl ester (BAEE) with Km values of 10.4 and 3.4 mM, respectively. The hydrolysis of BAEE was also inhibited by phenylarsonic acids. The kinetics of inhibition by m-nitrophenylarsonate were of the mixed type, and the K1 was 1.8 mM. The data followed a theoretical curve for a 1:1 enzyme-inhibitor complex with a dissociation constant of 1.8 mM. Inhibition by m-nitrophenylarsonate was pH dependent and followed a theoretical curve for the titration of a protonated group with a pKa of 7.0.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer A. N. Esteratic reactions catalyzed by subtilisins. J Biol Chem. 1967 Feb 10;242(3):433–436. [PubMed] [Google Scholar]
- Glazer A. N. Inhibition of "serine" esterases by phenylarsonic acids. alpha-chymotrypsin and the subtilisins. J Biol Chem. 1968 Jul 10;243(13):3693–3701. [PubMed] [Google Scholar]
- Glazer A. N. The time-dependent specific interation of 4-(4'-aminophenylazo)phenylarsonic acid with subtilsins. Proc Natl Acad Sci U S A. 1968 Mar;59(3):996–1002. doi: 10.1073/pnas.59.3.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGH R., RYSCHENKOW E. Pseudomonas maltophilia, an alcaligenes-like species. J Gen Microbiol. 1961 Sep;26:123–132. doi: 10.1099/00221287-26-1-123. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MORIHARA K., TSUZUKI H., OKA T., INOUE H., EBATA M. PSEUDOMONAS AERUGINOSA ELASTASE. ISOLATION, CRYSTALLIZATION, AND PRELIMINARY CHARACTERIZATION. J Biol Chem. 1965 Aug;240:3295–3304. [PubMed] [Google Scholar]
- McCullough J. L., Chabner B. A., Bertino J. R. Purification and properties of carboxypeptidase G 1 . J Biol Chem. 1971 Dec 10;246(23):7207–7213. [PubMed] [Google Scholar]
- Nakajima M., Mizusawa K., Yoshida F. Purification and properties of an extracellular proteinase of psychrophilic Escherichia freundii. Eur J Biochem. 1974 May 2;44(1):87–96. doi: 10.1111/j.1432-1033.1974.tb03460.x. [DOI] [PubMed] [Google Scholar]
- Prescott J. M., Wilkes S. H., Wagner F. W., Wilson K. J. Aeromonas aminopeptidase. Improved isolation and some physical properties. J Biol Chem. 1971 Mar 25;246(6):1756–1764. [PubMed] [Google Scholar]
- Smith E. L., Markland F. S., Kasper C. B., DeLange R. J., Landon M., Evans W. H. The complete amino acid sequence of two types of subtilisin, BPN' and Carlsberg. J Biol Chem. 1966 Dec 25;241(24):5974–5976. [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Verbruggen R., Duruisseau E., Baeck A. On the heterogeneity and the autodigestion of subtilisin "Carlsberg" as studied by agarose electrophoresis and Grabar-Williams immunoelectrophoresis. Biochim Biophys Acta. 1974 Sep 13;365(1):108–114. doi: 10.1016/0005-2795(74)90254-2. [DOI] [PubMed] [Google Scholar]



