Abstract
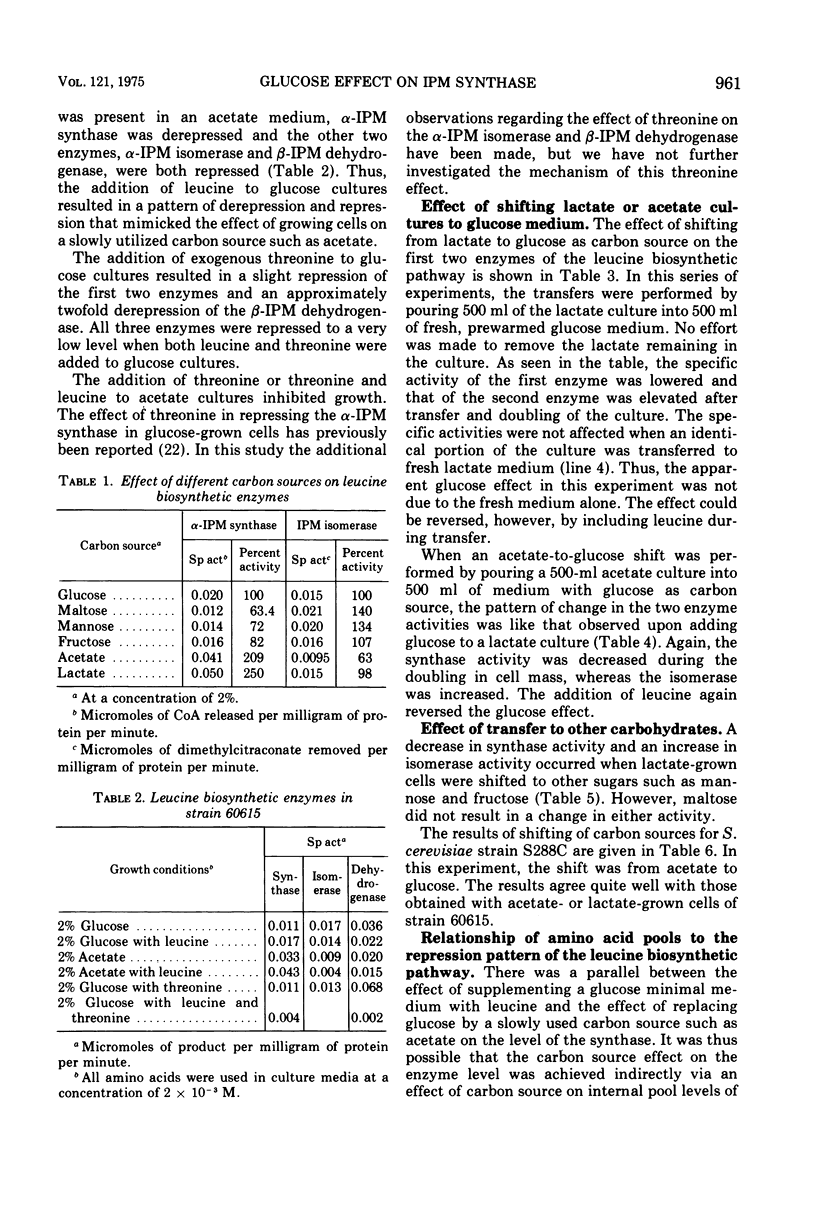
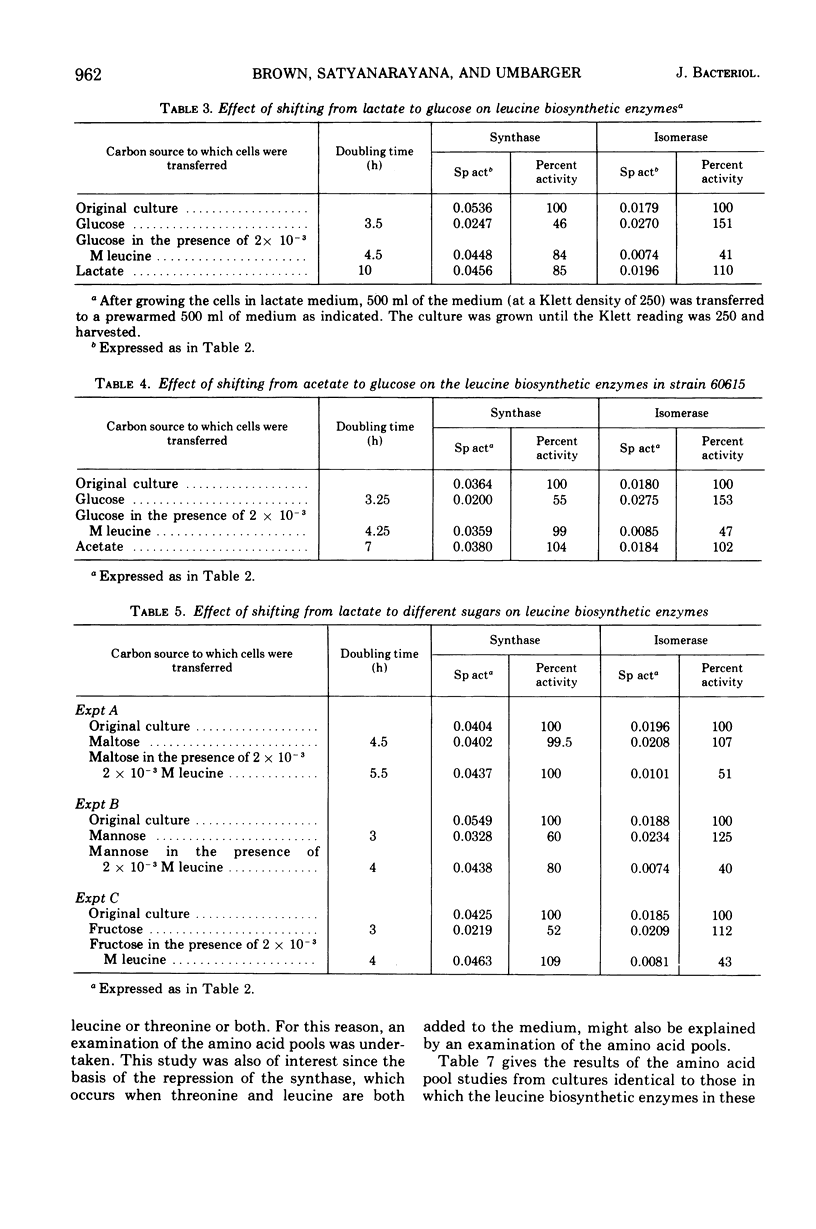
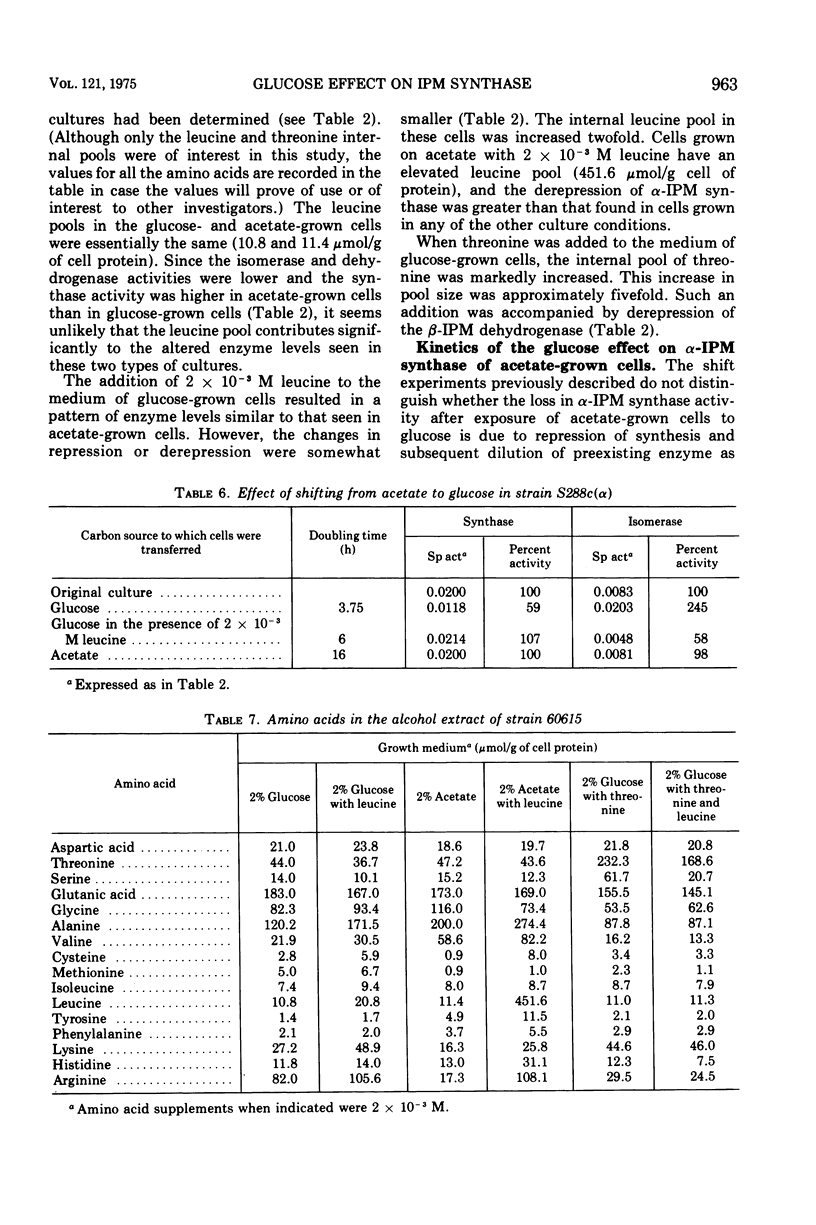
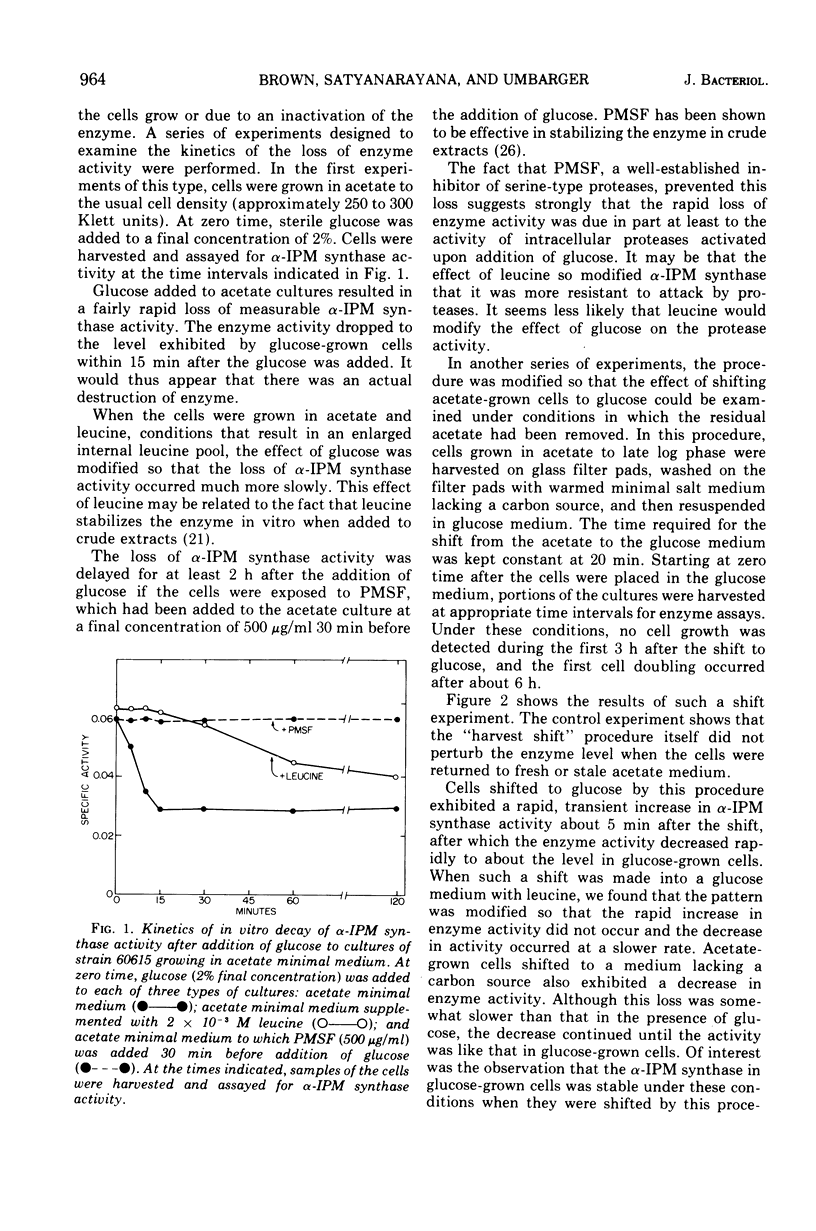
The three enzymes in the leucine biosynthetic pathway of yeast do not exhibit coordinate repression and derepression in response to the carbon source available in the culture medium. Growth in an acetate medium results in derepression of the first enzyme in the pathway, alpha-isopropylmalate synthase, and repression of the second two enzymes, alpha-isopropylmalate isomerase and beta-isopropylmalate dehydrogenase, relative to the levels found in glucose-grown cells. The role of endogenous leucine pools as a mediator of these differences was investigated. The leucine pools did not differ significantly between acetate-grown and glucose-grown cells. However, an elevated endogenous leucine pool, caused by exogenous leucine in the growth medium, did decrease the rate of decay of alpha-isopropylmalate synthase activity observed when acetate-grown cells were shifted to glucose. Evidence is provided suggesting that an elevated endogenous leucine pool may increase the in vivo stability of alpha-isopropylmalate synthase under several different conditions. Studies on the kinetics of alpha-isopropylmalate synthase decay in vivo and sensitivity to leucine inhibition indicate that there are two classes of the enzyme in acetate-grown yeast cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURNS R. O., UMBARGER H. E., GROSS S. R. THE BIOSYNTHESIS OF LEUCINE. III. THE CONVERSION OF ALPHA-HYDROXY-BETA-CARBOXYISOCAPROATE TO ALPHA-KETOISOCAPROATE. Biochemistry. 1963 Sep-Oct;2:1053–1058. doi: 10.1021/bi00905a024. [DOI] [PubMed] [Google Scholar]
- Bussey H., Umbarger H. E. Biosynthesis of branched-chain amino acids in yeast: regulation of synthesis of the enzymes of isoleucine and valine biosynthesis. J Bacteriol. 1969 May;98(2):623–628. doi: 10.1128/jb.98.2.623-628.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coukell M. B., Polglase W. J. Repression by glucose of acetohydroxy acid synthetase in Escherichia coli B. Biochem J. 1969 Feb;111(3):273–278. doi: 10.1042/bj1110273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duntze W., Neumann D., Holzer H. Glucose induced inactivation of malate dehydrogenase in intact yeast cells. Eur J Biochem. 1968 Jan;3(3):326–331. doi: 10.1111/j.1432-1033.1968.tb19533.x. [DOI] [PubMed] [Google Scholar]
- Epps H. M., Gale E. F. The influence of the presence of glucose during growth on the enzymic activities of Escherichia coli: comparison of the effect with that produced by fermentation acids. Biochem J. 1942 Sep;36(7-9):619–623. doi: 10.1042/bj0360619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson J. J., Jr, Boll M., Holzer H. Yeast malate dehydrogenase: enzyme inactivation in catabolite repression. Eur J Biochem. 1967 Mar;1(1):21–25. doi: 10.1007/978-3-662-25813-2_4. [DOI] [PubMed] [Google Scholar]
- Friedman S. B., Margolin P. Evidence for an altered operator specificity: catabolite repression control of the leucine operon in Salmonella typhimurium. J Bacteriol. 1968 Jun;95(6):2263–2269. doi: 10.1128/jb.95.6.2263-2269.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS S. R., BURNS R. O., UMBARGER H. E. THE BIOSYNTHESIS OF LEUCINE. II. THE ENZYMIC ISOMERIZATION OF BETA-CARBOXY-BETA-HYDROXYISOCAPROATE AND ALPHA-HYDROXY-BETA-CARBOXYISOCAPROATE. Biochemistry. 1963 Sep-Oct;2:1046–1052. doi: 10.1021/bi00905a023. [DOI] [PubMed] [Google Scholar]
- Gancedo C. Inactivation of fructose-1,6-diphosphatase by glucose in yeast. J Bacteriol. 1971 Aug;107(2):401–405. doi: 10.1128/jb.107.2.401-405.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. J. Metabolic regulation in glucose-limited chemostat cultures of Escherichia coli. J Bacteriol. 1970 Nov;104(2):698–706. doi: 10.1128/jb.104.2.698-706.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNGWIRTH C., GROSS S. R., MARGOLIN P., UMBARGER H. E. The biosynthesis of leucine. I. The accumulation of beta-carboxy-beta-hydroxyisocaproate by leucine auxotrophs of Salmonella typhimurium and Neurospora crassa. Biochemistry. 1963 Jan-Feb;2:1–6. doi: 10.1021/bi00901a001. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- MARGOLIN P. Genetic fine structure of the leucine operon in Salmonella. Genetics. 1963 Mar;48:441–457. doi: 10.1093/genetics/48.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz M., Getz G. S., Casey J., Swift H. Synthesis of mitochondrial and nuclear DNA in anerobically grown yeast during the development of mitochondrial function in response to oxygen. J Mol Biol. 1969 May 14;41(3):381–400. doi: 10.1016/0022-2836(69)90283-6. [DOI] [PubMed] [Google Scholar]
- Ryan E. D., Tracy J. W., Kohlhaw G. B. Subcellular localization of the leucine biosynthetic enzymes in yeast. J Bacteriol. 1973 Oct;116(1):222–225. doi: 10.1128/jb.116.1.222-225.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STADTMAN E. R. The coenzyme A transphorase system in Clostridium kluyveri. J Biol Chem. 1953 Jul;203(1):501–512. [PubMed] [Google Scholar]
- STRASSMAN M., CECI L. N. Enzymatic formation of alpha-isopropylmalic acid, an intermediate in leucine biosynthesis. J Biol Chem. 1963 Jul;238:2445–2452. [PubMed] [Google Scholar]
- Satyanarayana T., Umbarger H. E., Lindegren G. Biosynthesis of branched-chain amino acids in yeast: correlation of biochemical blocks and genetic lesions in leucine auxotrophs. J Bacteriol. 1968 Dec;96(6):2012–2017. doi: 10.1128/jb.96.6.2012-2017.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyanarayana T., Umbarger H. E., Lindegren G. Biosynthesis of branched-chain amino acids in yeast: regulation of leucine biosynthesis in prototrophic and leucine auxotrophic strains. J Bacteriol. 1968 Dec;96(6):2018–2024. doi: 10.1128/jb.96.6.2018-2024.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulm E. H., Böhme R., Kohlhaw G. Alpha-isopropylmalate synthase from yeast: purification, kinetic studies, and effect of ligands on stability. J Bacteriol. 1972 Jun;110(3):1118–1126. doi: 10.1128/jb.110.3.1118-1126.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. S., Neidhardt F. C. Synthesis and inactivation of aminoacyl-transfer RNA synthetases during growth of Escherichia coli. J Mol Biol. 1969 Aug 14;43(3):529–550. doi: 10.1016/0022-2836(69)90357-x. [DOI] [PubMed] [Google Scholar]


