Abstract
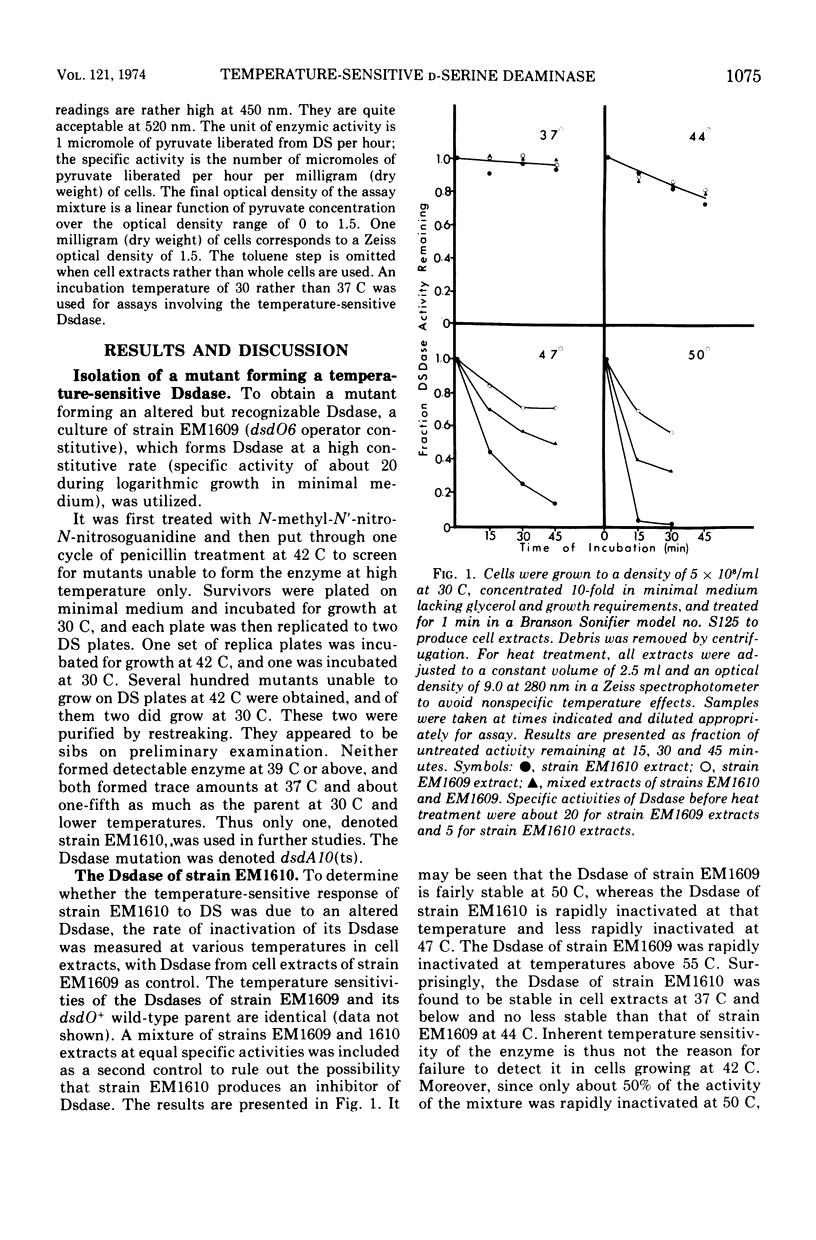
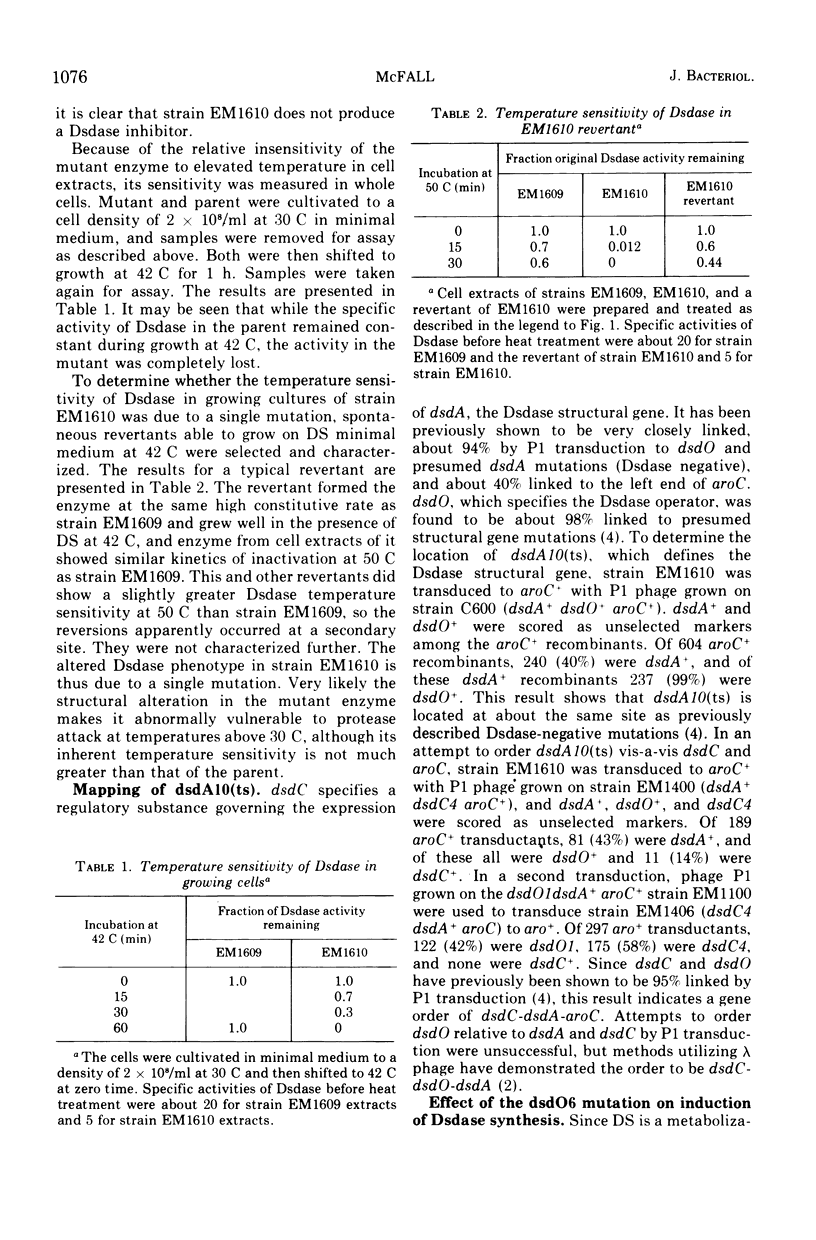
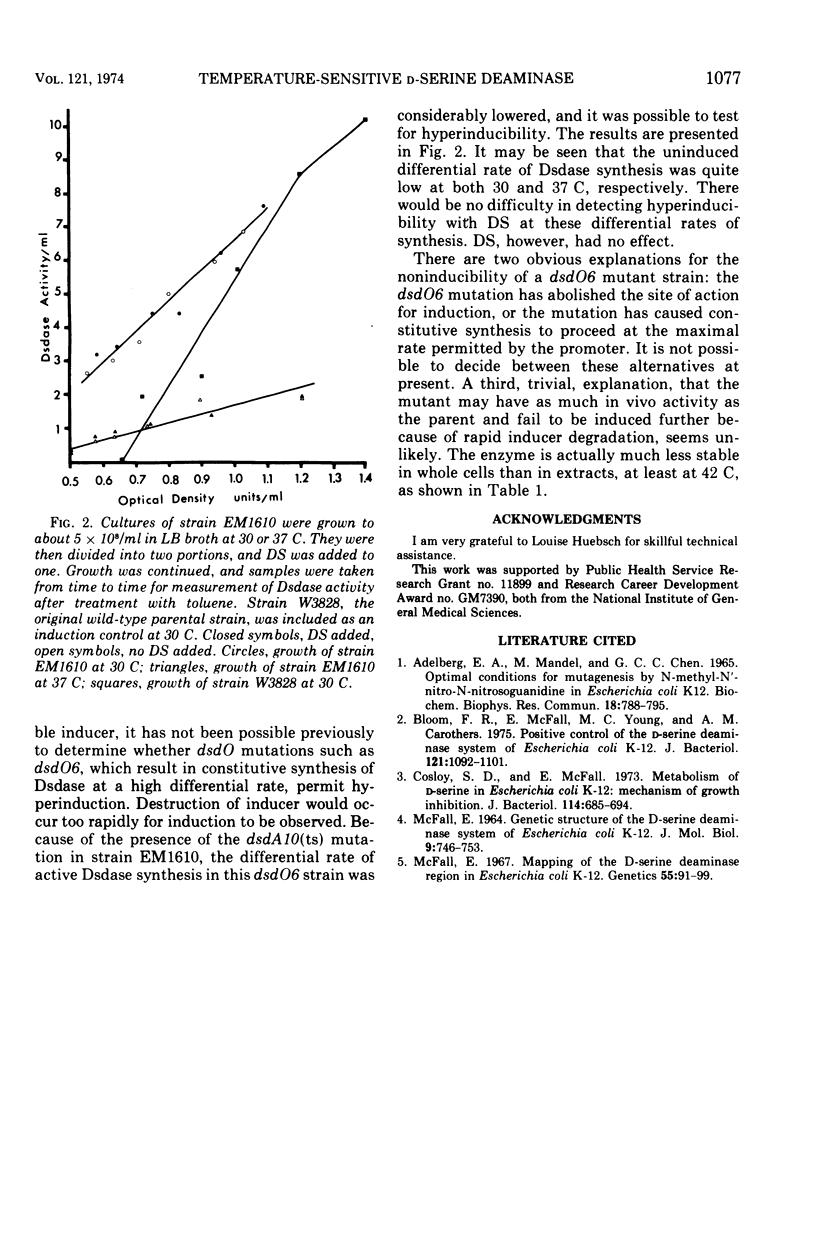
A single-site mutant of Escherichia coli K-12 able to grow in minimal medium in the presence of D-serine at 30 C but not at 42 C was isolated. The mutant forms a D-serine deaminase that is much more sensitive to thermal denaturation in vitro at temperatures above but not below 47 C than that of the wild type. No detectable enzyme is formed by the mutant at 42 C, however, and very little is formed at 37 C. The mutant enzyme is probably more sensitive to intracellular inactivation at high temperatures than the wild-type enzyme. The mutation lies in the dsdA region. The mutant also contains a dsdO mutation, which does not permit hyperinduction of D-serine deaminase synthesis.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom F. R., McFall E., Young M. C., Carothers A. M. Positive control in the D-serine deaminase system of Escherichia coli K-12. J Bacteriol. 1975 Mar;121(3):1092–1101. doi: 10.1128/jb.121.3.1092-1101.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosloy S. D., McFall E. Metabolism of D-serine in Escherichia coli K-12: mechanism of growth inhibition. J Bacteriol. 1973 May;114(2):685–694. doi: 10.1128/jb.114.2.685-694.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCFALL E. GENETIC STRUCTURE OF THE D-SERINE DEAMINASE SYSTEM OF ESCHERICHIA COLI. J Mol Biol. 1964 Sep;9:746–753. doi: 10.1016/s0022-2836(64)80179-0. [DOI] [PubMed] [Google Scholar]
- McFall E. Mapping of the d-serine deaminase region in Escherichia coli K-12. Genetics. 1967 Jan;55(1):91–99. doi: 10.1093/genetics/55.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]


