Abstract
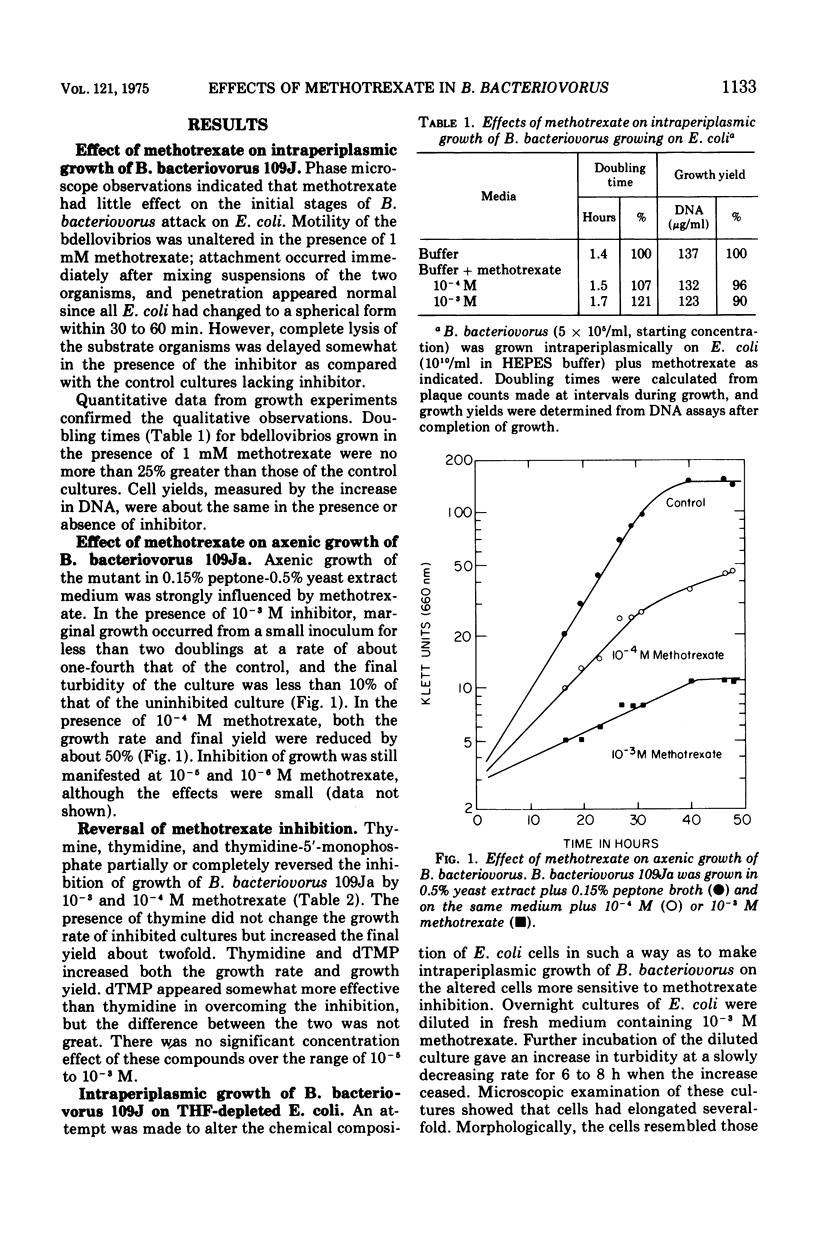
The intraperiplasmic growth rate and cell yield of wild-type Bdellovibrio bacteriovorus 109J, growing on Escherichia coli of normal composition as the substrate, were not markedly inhibited by 10-3 M methotrexate (4-amino-N10-methylpteroylglutamic acid). In contrast, the growth rate and cell yield of the mutant 109Ja, growing axenically in 0.5% yeast extract +0.15% peptone, were strongly inhibited by 10-4 and 10-3 M methotrexate. Thymine, thymidine, and thymidine-5'-monophosphate, in increasing order of effectiveness, partially or completely reversed the inhibition. E. coli depleted of tetrahydrofolate and having an abnormally high protein/deoxyribonucleic acid (DNA) ratio was obtained by growing it in the presence of methotrexate. B. bacteriovourus grew at a normal rate on these depleted E. coli cells but with somewhat reduced cell yield. Mexthotrexate (10-3 M) inhibited intraperiplasmic growth of bdellovibrio on the depleted E. coli somewhat more than it inhibited growth on normal E. coli, but the effects were small compared with inhibition of axenic growth of the mutant. Total bdellovibrio DNA after growth on the depleted E. coli in the presence or absence of methotrexate exceeded the initial quanity of E. coli DNA present. Thymidine-5'-monophosphate (10-3 M) largely reversed the inhibition and increased the amount of net synthesis of DNA. The data are consistent with the prediction that intraperiplasmic growth of B. bacteriovorus should be insensitive to all metabolic inhibitors that act by specifically preventing synthesis of essential monomers. The data also indicate that B. bacteriovorus possesses thymidylate synthetase, thymidine phosphorylase, and thymidine kinase, and has the potential to carry out de novo DNA synthesis from non-DNA precursors during intraperiplasmic growth. The results also suggest that methionyl tRNAfMet is not required for initiation of protein synthesis by B. bacteriovorus.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. S., Barner H. D. STUDIES ON UNBALANCED GROWTH IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1954 Oct;40(10):885–893. doi: 10.1073/pnas.40.10.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale B. A., Greenberg G. R. Effect of the folic acid analogue, trimethoprim, on growth, macromolecular synthesis, and incorporation of exogenous thymine in Escherichia coli. J Bacteriol. 1972 Jun;110(3):905–916. doi: 10.1128/jb.110.3.905-916.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerman H. W., Steers E., Jr, Redfield B. G., Weissbach H. Methionyl soluble ribonucleic acid transformylase. I. Purification and partial characterization. J Biol Chem. 1967 Apr 10;242(7):1522–1525. [PubMed] [Google Scholar]
- Diedrich D. L., Denny C. F., Hashimoto T., Conti S. F. Facultatively parasitic strain of Bdellovibrio bacteriovorus. J Bacteriol. 1970 Mar;101(3):989–996. doi: 10.1128/jb.101.3.989-996.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstadt J., Lengyel P. Formylmethionyl-tRNA dependence of amino acid incorporation in extracts of trimethoprim-treated Escherichia coli. Science. 1966 Oct 28;154(3748):524–527. [PubMed] [Google Scholar]
- Friedkin M. Thymidylate synthetase. Adv Enzymol Relat Areas Mol Biol. 1973;38:235–292. doi: 10.1002/9780470122839.ch5. [DOI] [PubMed] [Google Scholar]
- Harvey R. J. Growth and initiation of protein synthesis in Escherichia coli in the presence of trimethoprim. J Bacteriol. 1973 Apr;114(1):309–322. doi: 10.1128/jb.114.1.309-322.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hespell R. B., Rosson R. A., Thomashow M. F., Rittenberg S. C. Respiration of Bdellovibrio bacteriovorus strain 109J and its energy substrates for intraperiplasmic growth. J Bacteriol. 1973 Mar;113(3):1280–1288. doi: 10.1128/jb.113.3.1280-1288.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hespell R. B., Thomashow M. F., Rittenberg S. C. Changes in cell composition and viability of Bdellovibrio bacteriovorus during starvation. Arch Microbiol. 1974 May 20;97(4):313–327. doi: 10.1007/BF00403070. [DOI] [PubMed] [Google Scholar]
- Horowitz A. T., Kessel M., Shilo M. Growth cycle of predacious Bdellovibrios in a host-free extract system and some properties of the host extract. J Bacteriol. 1974 Jan;117(1):270–282. doi: 10.1128/jb.117.1.270-282.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenen J. G., Rittenberg S. C. Incorporation of long-chain fatty acids of the substrate organism by Bdellovibrio bacteriovorus during intraperiplasmic growth. J Bacteriol. 1975 Mar;121(3):1145–1157. doi: 10.1128/jb.121.3.1145-1157.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marcker K. The formation of N-formyl-methionyl-sRNA. J Mol Biol. 1965 Nov;14(1):63–70. doi: 10.1016/s0022-2836(65)80230-3. [DOI] [PubMed] [Google Scholar]
- Matin A., Rittenberg S. C. Kinetics of deoxyribonucleic acid destruction and synthesis during growth of Bdellovibrio bacteriovorus strain 109D on pseudomonas putida and escherichia coli. J Bacteriol. 1972 Sep;111(3):664–673. doi: 10.1128/jb.111.3.664-673.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miovic M., Pizer L. I. Effect of trimethoprim on macromolecular synthesis in Escherichia coli. J Bacteriol. 1971 Jun;106(3):856–862. doi: 10.1128/jb.106.3.856-862.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan G. A., Neuhard J. Pyrimidine metabolism in microorganisms. Bacteriol Rev. 1970 Sep;34(3):278–343. doi: 10.1128/br.34.3.278-343.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine M. J., Gordon B., Sarimo S. S. Protein initiation without folate in Streptococcus faecium. Biochim Biophys Acta. 1969 Apr 22;179(2):439–447. doi: 10.1016/0005-2787(69)90052-5. [DOI] [PubMed] [Google Scholar]
- Rittenberg S. C., Hespell R. B. Energy efficiency of intraperiplasmic growth of Bdellovibrio bacteriovorus. J Bacteriol. 1975 Mar;121(3):1158–1165. doi: 10.1128/jb.121.3.1158-1165.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenberg S. C., Langley D. Utilization of nucleoside monophosphates per Se for intraperiplasmic growth of Bdellovibrio bacteriovorus. J Bacteriol. 1975 Mar;121(3):1137–1144. doi: 10.1128/jb.121.3.1137-1144.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenberg S. C. Nonidentity of Bdellovibrio bacteriovorus strains 109D and 109J. J Bacteriol. 1972 Jan;109(1):432–433. doi: 10.1128/jb.109.1.432-433.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenberg S. C., Shilo M. Early host damage in the infection cycle of Bdellovibrio bacteriovorus. J Bacteriol. 1970 Apr;102(1):149–160. doi: 10.1128/jb.102.1.149-160.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOLP H., STARR M. P. BDELLOVIBRIO BACTERIOVORUS GEN. ET SP. N., A PREDATORY, ECTOPARASITIC, AND BACTERIOLYTIC MICROORGANISM. Antonie Van Leeuwenhoek. 1963;29:217–248. doi: 10.1007/BF02046064. [DOI] [PubMed] [Google Scholar]
- Samuel C. E., Murray C. L., Rabinowitz J. C. Methionine transfer ribonucleic acid from folate-sufficient and folate-deficient Streptococcus faecalis R. J Biol Chem. 1972 Nov 10;247(21):6856–6865. [PubMed] [Google Scholar]
- Seidler R. J., Starr M. P. Isolation and characterization of host-independent Bdellovibrios. J Bacteriol. 1969 Nov;100(2):769–785. doi: 10.1128/jb.100.2.769-785.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilo M., Bruff B. Lysis of Gram-negative bacteria by host-independent ectoparasitic Bdellovibrio bacteriovorus isolates. J Gen Microbiol. 1965 Sep;40(3):317–328. doi: 10.1099/00221287-40-3-317. [DOI] [PubMed] [Google Scholar]
- Starr M. P., Baigent N. L. Parasitic interaction of Bdellovibrio bacteriovorus with other bacteria. J Bacteriol. 1966 May;91(5):2006–2017. doi: 10.1128/jb.91.5.2006-2017.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varon M., Shil M. Interacton of Bdellovibrio bacteriovorus and host bacteria. I. Kinetic studies of attachment and invasion of Escherichia coli B by Bdellovibrio bacteriovorus. J Bacteriol. 1968 Mar;95(3):744–753. doi: 10.1128/jb.95.3.744-753.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]


