Abstract
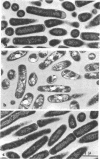
Cells of Escherichia coli strain B develop large intracellular vacuoles and exhibit an abnormally long lag phase when inoculated into a defined medium to glucose and salts containing 3 times 10-6 M Cd2+. Early in this lag, about 95% of the cells fail to form colonies when plated on nutrient broth-NaCl-agar. Prior to the initiation of proliferation, the morphology of these cells becomes normal. They regain viability in the absence of deoxyribonucleic acid replication. The rate and extent of growth are normal once proliferation begins. This reversible phenomenon of accommodation to a growth-inhibiting concentration of Cd2+ does not appear to result from a selection of mutant cells. Cells which are proliferating in the presence of Cd2+ accumulate the ion to a very high concentration. In membranes and 31% in the cytoplasm. In unaccommodated cells, the figures are 2%, 75%, and 23%, respectively. The activity of alkaline phosphatase, a zinc-metalloenzyme which is inhibited by cadmum and is located between cell wall and membrane, is not abnormally low in accommodated cells, suggesting that the cadmim is compartmentalized in these cells. Molecular sieve chromatography of cell extracts shows that the Cd2+ is associated with two classes of macromolecules. It appears that accommodation of E. coli to the presence of Cd2+ involves exclusion of the ion from the cell and reversal of damage caused by prior exposure to the ion.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applebury M. L., Coleman J. E. Escherichia coli alkaline phosphatase. Metal binding, protein conformation, and quaternary structure. J Biol Chem. 1969 Jan 25;244(2):308–318. [PubMed] [Google Scholar]
- Esfahani M., Limbrick A. R., Knutton S., Oka T., Wakil S. J. The molecular organization of lipids in the membrane of Escherichia coli: phase transitions. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3180–3184. doi: 10.1073/pnas.68.12.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G. W., Majors P. F., Cornatzer W. E. Mechanism for cadmium and zinc antagonism of copper metabolism. Biochem Biophys Res Commun. 1970 Sep 10;40(5):1142–1148. doi: 10.1016/0006-291x(70)90913-7. [DOI] [PubMed] [Google Scholar]
- FRASCA J. M., PARKS V. R. A ROUTINE TECHNIQUE FOR DOUBLE-STAINING ULTRATHIN SECTIONS USING URANYL AND LEAD SALTS. J Cell Biol. 1965 Apr;25:157–161. doi: 10.1083/jcb.25.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambleton P. Repair of wall damage in Escherichia coli recovered from an aerosol. J Gen Microbiol. 1971 Nov;69(1):81–88. doi: 10.1099/00221287-69-1-81. [DOI] [PubMed] [Google Scholar]
- KAGI J. H., VALEE B. L. Metallothionein: a cadmium- and zinc-containing protein from equine renal cortex. J Biol Chem. 1960 Dec;235:3460–3465. [PubMed] [Google Scholar]
- Kaback H. R. The role of the phosphoenolpyruvate-phosphotransferase system in the transport of sugars by isolated membrane preparations of Escherichia coli. J Biol Chem. 1968 Jul 10;243(13):3711–3724. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lazdunski C., Petitclerc C., Lazdunski M. Structure-function relationships for some metalloalkaline phosphatases of E. coli. Eur J Biochem. 1969 Apr;8(4):510–517. doi: 10.1111/j.1432-1033.1969.tb00556.x. [DOI] [PubMed] [Google Scholar]
- Lucis O. J., Lucis R., Shaikh Z. A. Cadmium and zinc in pregnancy and lactation. Arch Environ Health. 1972 Jul;25(1):14–22. doi: 10.1080/00039896.1972.10666127. [DOI] [PubMed] [Google Scholar]
- Mitra R. S., Bernstein I. A. Effects of atractyloside on the incorporation of radioactive precursors into DNA in isolated rat liver mitochondria. Arch Biochem Biophys. 1970 Dec;141(2):519–524. doi: 10.1016/0003-9861(70)90169-4. [DOI] [PubMed] [Google Scholar]
- Mullinix K. P., Rosenkranz H. S. Recovery from N-hydroxyurethan-induced death. J Bacteriol. 1971 Feb;105(2):565–572. doi: 10.1128/jb.105.2.565-572.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Potter V. R. Intracellular responses to environmental change: the quest for optimum environment. Environ Res. 1970 Mar;3(2):176–186. doi: 10.1016/0013-9351(70)90014-9. [DOI] [PubMed] [Google Scholar]
- Pulido P., Kägi J. H., Vallee B. L. Isolation and some properties of human metallothionein. Biochemistry. 1966 May;5(5):1768–1777. doi: 10.1021/bi00869a046. [DOI] [PubMed] [Google Scholar]
- Shaikh Z. A., Lucis O. J. Cadmium and zinc binding in mammalian liver and kidneys. Arch Environ Health. 1972 Jun;24(6):419–425. doi: 10.1080/00039896.1972.10666118. [DOI] [PubMed] [Google Scholar]
- Tornabene T. G., Edwards H. W. Microbial uptake of lead. Science. 1972 Jun 23;176(4041):1334–1335. doi: 10.1126/science.176.4041.1334. [DOI] [PubMed] [Google Scholar]
- Webb M. Protection by zinc against cadmium toxicity. Biochem Pharmacol. 1972 Oct 15;21(20):2767–2771. doi: 10.1016/0006-2952(72)90024-x. [DOI] [PubMed] [Google Scholar]
- Winge D. R., Rajagopalan K. V. Purification and some properties of Cd-binding protein from rat liver. Arch Biochem Biophys. 1972 Dec;153(2):755–762. doi: 10.1016/0003-9861(72)90395-5. [DOI] [PubMed] [Google Scholar]