Abstract
Estrogen has long been known to play an important role in coordinating the neuroendocrine events that control sexual development, sexual behavior and reproduction. Estrogen actions in other, non-reproductive areas of the brain have also been described. It is now known that estrogen can also influence learning, memory, and emotion and has neurotrophic and neuroprotective properties. The actions of estrogen are largely mediated through at least two intracellular estrogen receptors. Both estrogen receptor-alpha and estrogen receptor-beta are expressed in a wide variety of brain regions. Estrogen receptor-alpha (ERα), however, undergoes developmental and brain region-specific changes in expression. The precise molecular mechanisms that regulate its expression at the level of gene transcription are not well understood. Adding to the complexity of its regulation, the estrogen receptor gene contains multiple promoters that drive its expression. In the cortex in particular, the ERα mRNA expression is dynamically regulated during postnatal development and again following neuronal injury. Epigenetic modification of chromatin is increasingly being understood as a mechanism of neuronal gene regulation. This review examines the potential regulation of the ERα gene by such epigenetic mechanisms.
Introduction
Estrogens, and in particular, 17β-estradiol (E2), the primary biologically active form of estrogen, have long been known to play a crucial role in coordinating the neuroendocrine events that control sexual development, sexual behavior and reproduction. In rodents, E2 is critical for sexual differentiation of the brain (see review by Gore in this issue and [1]. Generation of the differences between the male and female brain results from the exposure of the male brain to E2 [2]. During early postnatal development, the female brain is believed to be isolated from E2 by the presence of alpha-fetoprotein that prevents circulating E2 from crossing the blood brain barrier [3]. The effects of E2 on neural circuits and apoptosis of neurons lead to long-term differences in the male and female brain [4; 5; 6]. In addition to its role in development, E2 modulates numerous facets of brain function in the adult brain (for review see [7]). Such actions include protection against neuronal injury, involvement in learning and memory as well as promoting the formation of synapses [8; 9; 10; 11; 12].
The majority of these crucial and diverse actions of E2 require the action of an estrogen receptor, whether it be in a classical nuclear manner or mediated by cytoplasmic or membrane mechanisms. Indeed, E2 binding studies have shown a unique binding pattern in non-reproductive brain areas such as the cortex and hippocampus [13; 14]. Furthermore, these binding patterns change during postnatal development and decrease dramatically as the animal approaches puberty [15]. While the molecular actions of steroid hormones mediated through their receptors have been extensively studied in the brain and in peripheral tissues, considerably less is known about the molecular mechanisms that regulate the expression of steroid hormone receptors themselves. Because of the dramatic and dynamic regulation of estrogen receptor-alpha (ERα) mRNA described below, and its important role in numerous neuronal processes, this review will focus on the regulation of expression of the ERα gene in several brain regions and present potential molecular mechanisms that control its expression.
Estrogen Receptor Expression
The physiological effects resulting from E2 actions in target tissues are mediated by changes in the expression patterns of specific target genes. Many of these actions are mediated by intracellular receptors, and to date, two nuclear estrogen receptors have been well characterized, ERα and ERβ [16; 17; 18; 19; 20]. Additionally, membrane receptors are believed to mediate rapid actions of estrogens under certain physiological conditions [21; 22; 23; 24]. Both ERα and ERβ have been detected in both neurons and glia in the brain [25; 26] and are expressed throughout the brain, albeit with distinct patterns of expression. The distribution of the expression of protein and mRNA for ERα and ERβ have been detailed elsewhere [27; 28; 29; 30]. In general, the protein expression patterns often match the mRNA expression patterns [31; 32]. Examples exist, however, where ER protein levels do not correlate with ERα mRNA expression [15; 33; 34]. Whether these differences reflect alterations in post-transcriptional processing or translation remain to be seen. Alternatively, the sensitivity of some of the methods utilized to identify protein levels have not be sufficient to observe subtle differences in protein expression.
Significant homology exists between the human, mouse and rat genes, at both RNA and protein levels. In all species, the ERα gene is preceded by multiple promoters which generate several mRNA splice variants (Figure 1 modified from [35] and [36]). Alternative splicing occurs at the first exon of each promoter, which is then spliced to a common splice acceptor site upstream of the translational initiation codon in Exon 1 the ERα gene. This alternative promoter splicing results in mRNA splice variants that differ only in their 5’ untranslated region. The existence of these different RNAs is thought to be important in regulating stability or processing of the mRNA [35]. The regulatory binding sites of transcription factors that control gene expression are likely upstream of these exons, but may also be contained within the untranslated regions. All variants, however, produce the same protein suggesting that this differential promoter usage may be important for tissue or development-specific gene regulation.
Figure 1.
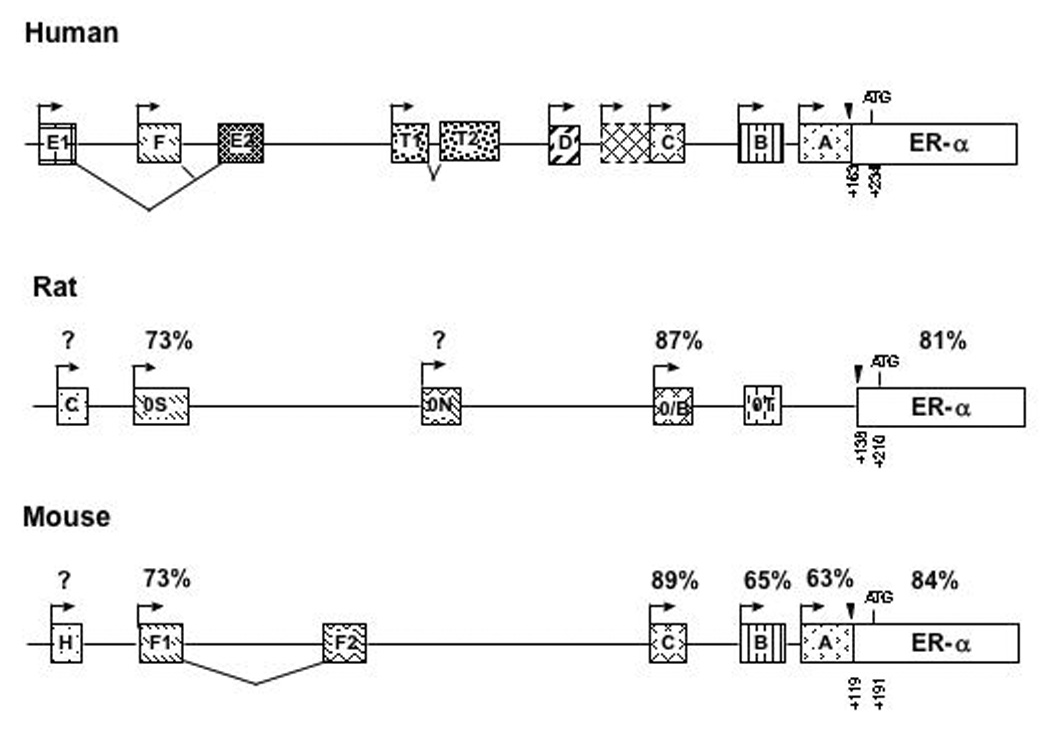
Schematic representation of the promoter regions of the human, rat and mouse ERα gene (modified from [35] and [36]). The boxes represent exons of the ERα gene. The first exon that encodes the ERα protein is represented by the white box containing “ERα”. The arrows represent transcriptional start sites and the translational start site is shown (ATG). The stippled boxes represent the upstream exons that encode for different 5’ untranslated regions of the ERα mRNA, driven by different promoters. Exons that do not contain a promoter are spliced together (lines under the boxes). The black triangles indicate the common splice site to which all upstream exons are spliced to the exon that encodes the ERα protein. All nomenclature regarding the naming of the promoters in this review is based on the nomenclature originally proposed by Kos, et al. [35] with the addition of the rat promoter (0T) as described in [36]. The percentages above the ERα protein refer to the percent homology compared to humans.
The human estrogen receptor gene contains at least seven upstream promoters that can initiate transcription [35] (Figure 1). Promoters A and C are expressed in normal and cancerous breast and uterine tissue [37; 38; 39]. Promoter E1/E2 is utilized in human liver [40; 41] and promoter F is utilized to drive ERα expression in osteoblasts [42]. In the brain, distinct patterns of promoter usage have been detected by in situ hybridization [27]. Promoter A is associated with areas of the human brain that express low, constitutive levels of ERα mRNA including the supraoptic nucleus, diagonal band of Broca, amygdala, and hippocampus (CA3 and dentate gyrus). Promoter B is utilized in restricted areas where ERα mRNA is more highly expressed including the arcuate nucleus of the hypothalamus. Additionally, low levels of promoter B are detected in Layer V of the temporal cortex in humans.
In rats, at least four upstream promoters have been identified [43; 44; 45]. The predominant exons include C, 0S, 0N and 0/B. 0/B is equivalent to promoter C in humans (Figure 1) and is expressed in the anterior pituitary, hypothalamus, amygdala as well as uterus and ovary. 0N is utilized in the liver [45]. A promoter equivalent to the human promoter A does not exist in the rat [45]. In transgenic rats engineered to express GFP under the control of the 0/B promoter, expression is observed in the preoptic area, bed nucleus of the stria terminalis, arcuate nucleus and medial amygdala [46]. 0/B driven expression was also detected in the cortex and hippocampus. In the neonatal rat cortex 0/B and 0S are both utilized [47]. Additionally, promoter 0T has been described in rats, and while it is present in the brain, its function is not known [48].
The mouse ERα gene is preceded by at least six promoters (A, B, C, F1, F2 and H) generating five mRNA splice variants [49]. F1/F2 are spliced together to produce a single transcript. The ERα promoters are differentially expressed among various cell types and tissues [49]. A, C, and F are widely expressed in the uterus, brain, kidney, and muscle. Promoter H is utilized exclusively in liver, while Promoter C dominates ERα expression in the whole brain of adult mice. Recently, we have examined the promoter expression in the neonatal cortex of mice by RT-PCR using primers specific to the different mouse promoters [49] [50] (Figure 2). The timepoints examined correlate with dramatic changes in ERα mRNA expression as the animal approaches puberty [51; 52]. All promoters were utilized in the cortex of both male and female mice throughout postnatal life. These experiments were performed in triplicate. Quantification of the relative intensity of the PCR products did not demonstrate significant differences the utilization of the promoters across development. Additionally, although it is not possible to make direct comparisons between PCR products from different primer sets, it appears that all are expressed at relatively comparable levels in the developing cortex. This observation would suggest that differential usage of the known promoters is not responsible for the developmental changes in ERα mRNA observed in the cortex [51; 52]. It remains possible, however, that other unknown brain-specific promoters exist that have not yet been identified that contribute to the regulation of ERα mRNA expression. Identification of such unknown promoter regions will lead to new potential players in understanding how the ERα gene is regulated at the molecular level.
Figure 2.
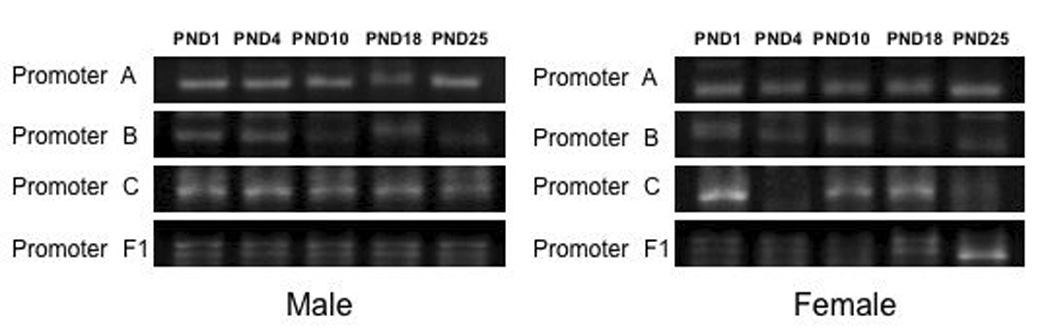
Known ERα promoters are present in the isocortex during postnatal development in both male (Left) and female mice (Right). RT-PCR was performed on RNA isolated from postnatal day (PND) 1, PND4, PND10, PND18 and PND25 mouse cortex utilizing promoter specific primers [49]. These days represent various times during postnatal development in which ERα is dynamically regulated leading to puberty. PCR products were separated by agarose gel electrophoresis and visualized with ethidium bromide. A representative micrograph of the PCR products are shown. A positive control with the housekeeping gene, His3.1 was also performed (data not shown). All experiments were repeated three times with an n=3.
Regulation of Estrogen Receptor mRNA
Developmental regulation of estrogen receptor-alpha
Dynamic changes take place throughout development in ERα protein levels that are also reflected by changes in ERα mRNA expression [15; 29; 53]. Autoradiographic studies first demonstrated high levels of estradiol binding in non-hypothalamic regions such as the cortex and hippocampus during the first two weeks of life [13; 54; 55]. Interestingly, this expression declines as animals approach puberty. In rats, ERα mRNA expression was also shown to correlate with the changes in estrogen binding in the hippocampus [56]. Similar changes in ERα mRNA expression during the first three weeks of postnatal life have also recently been shown in the cortex of mice [52]. By the time animals reach adulthood, however, ERα mRNA is expressed at very low levels in the mouse cortex.
The factors that control this developmental decline in ERα mRNA expression are not known. The triggers, however, are believed to be intrinsic to the cells and independent of external influences [57; 58]. Studies by O’Keefe and colleagues demonstrated that when either rat fetal hippocampal or cortical tissue was transplanted to the brain of a neonatal animal, the developmental profile of ERα mRNA expression in both the hippocampal and the cortical transplants continued with the profile of the age of the donor. This suggests that the control of the change in ERα gene expression is programmed in the developing tissue and does not rely on cues from the surrounding tissue. Similar results were observed with transplants from the hypothalamus transplanted to animals treated with different hormonal paradigms [57], suggesting that hormonal signals do not regulate ER expression.
To begin to characterize these changes further, we have recently employed in an in vitro model utilizing organotypic explants cultures of neonatal cortex to study the developmental changes in ERα mRNA expression. These cultures consist of 300 µM thick slices of the isocortex taken from postnatal day 3 mice that are allowed to grow in vitro for up to three weeks. Over this time, the same changes in ERα mRNA expression that are observed in intact animals are also observed in vitro (Figure 3). Control experiments have shown that these changes are specific to ERα mRNA. These data appear to confirm that the signal that causes the decline is not the result of extrinsic factors or synaptic inputs from other brain regions, but rather either the loss of a stimulatory signal or intrinsic to the cells themselves.
Figure 3.
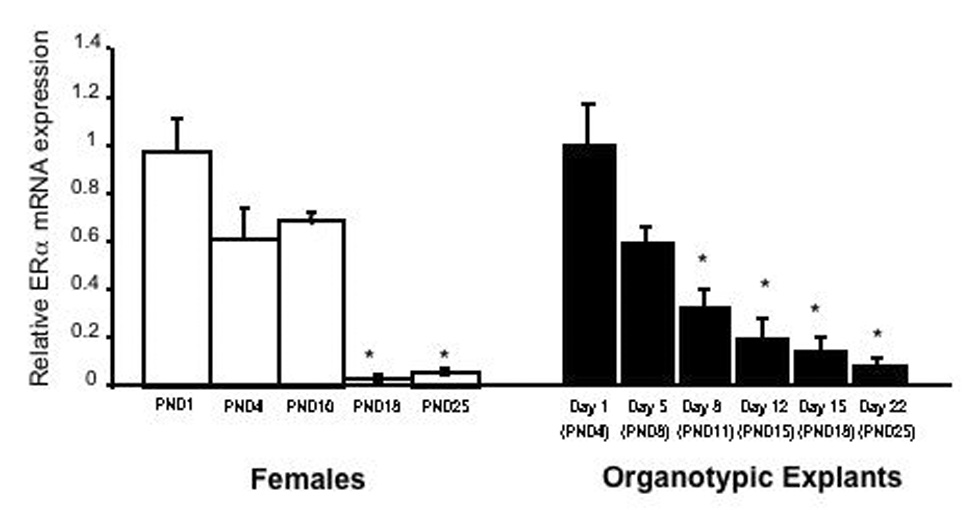
Developmental decline in ERα mRNA expression in the mouse cortex both in vivo (Left) and in vitro (Right). (Left) Quantitative real-time PCR was performed on total RNA isolated from the isocortex of PND1, PND4, PND10, PND18 and PND25 female mice. Data were normalized to the housekeeping gene Histone 3.1 and expressed relative to PND1. (Right) ERα mRNA expression in vitro follows in vivo trends across developmental time points. Quantitative real-time PCR utilizing ERα specific primers was performed on RNA isolated from organotypic explants from PND3 females kept in culture for varying lengths of time. Explant cultures contain neurons and glia of the cortex in the absence of other brain regions. The corresponding postnatal days to the days in vitro are shown on the X- axis. Data were normalized to the housekeeping gene Histone 3.1 and expressed relative to PND1. Bars represent the mean +/− SEM, n=4. * = significantly different from PND1. (Redrawn from [52]).
Regulation of estrogen receptor-alpha mRNA in the adult
In the adult brain, E2 can regulate its own receptor gene expression. ERα mRNA levels have been shown to fluctuate with the estrous cycle with levels highest on estrus and lowest on proestrus [59], suggesting E2 decreases ERα mRNA expression. Furthermore, ovariectomy results in increased expression of ERα mRNA levels in several brain areas including the medial preoptic nucleus, arcuate nucleus and ventromedial nucleus of the hypothalamus [59; 60]. This relationship does not hold true in all brain regions or in both genders suggesting that other factors may interact to control ERα mRNA expression in addition to simply E2. A similar phenomenon has also been observed in humans where E2 appears to differentially regulate ERα mRNA expression in specific hypothalamic nuclei [61; 62]. Women have more ER-expressing cells in the diagonal band of Broca, suprachiasmatic nucleus and the ventromedial nucleus of the hypothalamus, while there appears to be less of an effect of E2 in other nuclei such as the medial preoptic area, paraventricular nucleus and lateral hypothalamus. Whether these gender differences in expression reflect a different response to gonadal hormones in the adult, or an underlying sex difference in the brain, remains to be seen.
In adult female rodents, ERα mRNA expression, which is virtually absent in the adult, is reactivated in the cortex following neuronal injury such as stroke [63]. Middle cerebral artery occlusion (MCAO) is a well-established model of focal ischemia in rodents. Early studies demonstrated a striking gender difference in neuronal cell death following MCAO [64; 65; 66]. Pretreatment with even low doses of E2 is sufficient to exert dramatic neuroprotection in the brains of both female and male rats [63; 65; 67]. MCAO results in a loss of blood flow injuring the striatum and overlying cortex, causing primarily apoptotic cell death in the cortex [68]. E2 has been shown to prevent this cell death in the cortex and this protection is dependent on the presence of ERα [69; 70]. Following MCAO, the ERα gene in the cortex is rapidly increased from nearly undetectable levels to high levels within hours of the onset of the injury [71]. Although some slight differences are observed in the timecourse of expression, this activation is independent of E2 treatment. Recently it has been demonstrated that this increase in ERα mRNA expression is specific to females, as males do not show a similar increase [72]. These data indicate that following a noxious stimulus such as stroke, neurons may revert back to a developmental expression pattern of gene expression, perhaps as a compensatory mechanism to prevent cell death. As a result, ERα mRNA and protein are upregulated in females and cell death can be prevented. Understanding mechanisms of ERα mRNA regulation during development and following a stimulus such as neuronal injury, is critical for identifying a potential molecular target for mediating the neuroprotective actions of E2.
Additionally, a similar phenomenon occurs in an in vitro model of stroke. A chemical model of ischemia (potassium cyanide/2-deoxyglucose) that models the metabolic environment of ischemia in MCAO has been successfully used in organotypic explants from the cortex to induce apoptotic cell death [73; 74]. In organotypic cultures that have been grown in culture for two weeks and thus, lack ERα mRNA expression (Figure 3), this metabolic injury also stimulates an increase in ERα mRNA expression as measured by RT-PCR [75]. Taken together, these data suggest that cellular factors are activated in response to neuronal injury to influence ERα mRNA expression. Furthermore, these signals are not hormonally or synaptically mediated as ERα mRNA is also activated in isolated cortical cultures.
Molecular regulators of ERα gene expression
Very little is known about the molecular factors that directly regulate the control of ERα mRNA expression [76]. Some extracellular signals have been shown to in inhibit ERα expression in breast and ovarian tissue [77; 78; 79; 80]. In particular progesterone, vitamin D, and insulin can all decrease ERα mRNA expression in these tissues. Additionally, epidermal growth factor and gonadotropin releasing hormone have been shown to suppress ERα mRNA expression in the ovary and in MCF breast cancer cells [81; 82]. Aside from E2 as described in specific areas of the hypothalamus above, very little has been shown to increase ERα mRNA expression in the brain [76]. Little is known about the regulatory elements in the promoter regions of ERα. One notable exception is the study of the role of the Stat5 consensus sequence in the 0/B promoter of the rat [83]. Stat5 is a transcriptional activator protein involved in the JAK2-Stat5 pathway that has shown to be activated by prolactin, resulting in increased expression of ERα in the uterus and in the medial preoptic area of the hypothalamus [84]. Inhibition of Stat5 binding abolishes the ability of prolactin to increase ERα gene expression [85]. While the possibility of these factors in the brain regulating ERα mRNA in a similar fashion during development or after injury cannot be ruled out, it appears that additional mechanisms of ERα mRNA regulation in the brain need to be investigated. This is likely particularly true to enhance our understanding of the developmental differences in expression observed in a brain-region specific manner.
Epigenetic Regulation of Estrogen Receptor-alpha Gene Expression
At the molecular level, epigenetic modification of chromatin involves chemical changes to the DNA and associated proteins (for review see [86] and [87]). These modifications are heritable and can be passed to daughter cells during mitosis or meiosis [88]. Epigenetic modifications include DNA methylation, histone methylation and histone acetylation [89; 90]. Histones can also be ubiquitinated and phosphorylated leading to epigenetic regulation of gene expression where methylated DNA and condensed chromatin result in decreased gene expression [86]. DNA methylation can suppress transcription directly by recruiting binding proteins that interfere with basal transcriptional machinery or can recruit histone deacetylases that further compact the chromatin and thus suppress gene transcription [88](Figure 4). Although neurons do not divide, epigenetic modification of chromatin in neurons plays an important role in regulating gene expression during neuronal development and in learning and memory [91; 92]. Although histone modifications also likely play a role as well in the epigenetic regulation of gene expression, this review will focus on the epigenetic modification of DNA methylation.
Figure 4.
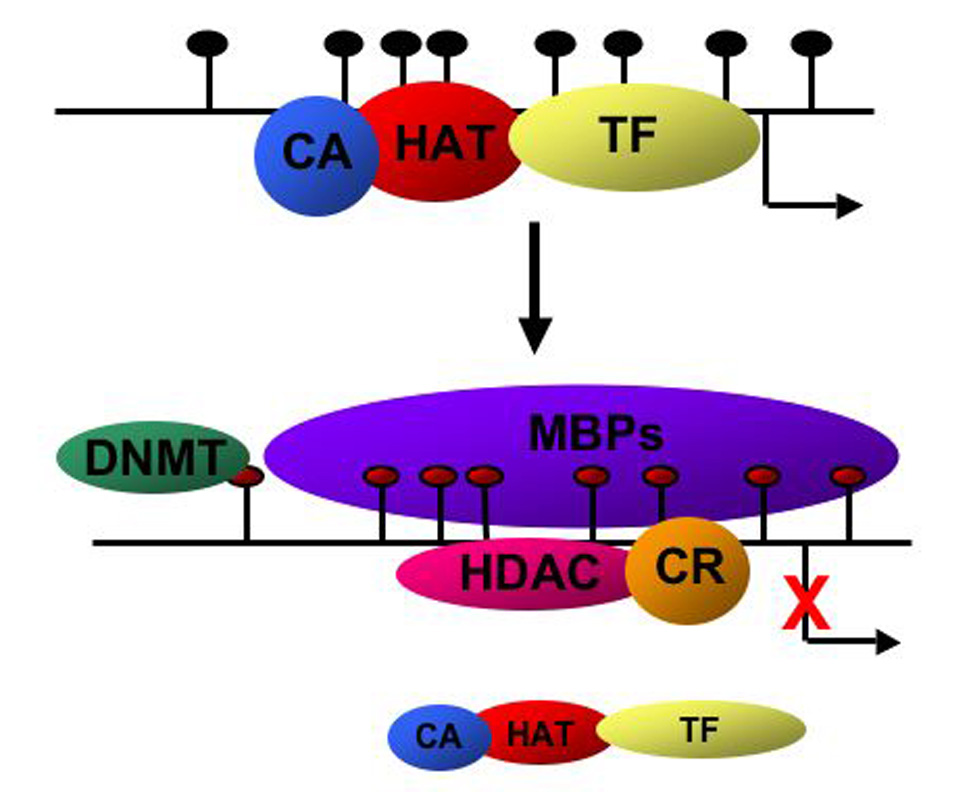
Schematic diagram of DNA methylation in regulating transcriptional activity. CpG islands in the promoter regions are represented as black circles. Under active transcriptional conditions, histone acetyltransferases (HAT) co-activators (CA) and the basal transcriptional machinery (TF) all interact at the promoter of active genes. Following methylation of the cytosines in CpG islands (red circles) by DNA methyltransferases (DNMT), methyl-DNA binding proteins (MBPs) bind to the methylated DNA, recruit histone deacetylases (HDAC) and co-repressors (CR) to the promoter. Transcription of the target gene is inhibited and CA, HAT and TFs no longer interact with the promoters.
The first step in DNA methylation involves the enzymatic transfer of a methyl group to the 5-position of the pyrimidine ring of a cytosine residue that is followed by a guanine (CpG dinucleotides). These modifications of the cytosines in CpG islands are carried out initially by DNA methyl transferase 3A (DNMT3A) [87]. Methylated cytosines are then recognized by methyl-CpG-binding proteins that inhibit transcription by interfering with the normal transcriptional machinery [90]. These CpG islands can be found upstream or downstream of the transcriptional start site. The family of methyl-CpG-binding proteins includes methyl binding domain (MBD) proteins 1, 2, 3, 4 and MeCP2 [93; 94; 95]. These proteins also associate with co-repressor complexes that include histone deacetylases (HDAC) [96; 97]. Together, these complexes suppress transcription of genes with methylated promoter DNA.
Peripheral Tissues
Methylation of the ERα promoter has been reported to occur as a direct function of physiological aging in the normal human colon [98] and as part of a pathological progression of numerous types of cancerous tissues [98; 99; 100; 101; 102; 103; 104]. Although evidence exists for multiple mechanisms of epigenetic modification of the ERα gene, DNA methylation has been the most widely described epigenetic phenomenon.
Up to one third of breast cancers that initially express ERα, lose ERα expression during tumor progression [105]. The prognosis and treatment are poor for ERα -negative tumors due to a lack of effective endocrine therapies [106; 107]. In a significant fraction of breast cancers, the absence of ERα RNA expression is a result of aberrant methylation of CpG islands in the promoter areas located in the 5’ regulatory regions of the ERα gene [104; 108]. The methylation of ERα promoters in breast cancer cell lines can be reversed by the administration of a DNMT inhibitor that reverses methylation resulting in the re-expression of ERα mRNA expression. In breast cancer cells, all MBD proteins and MeCP2 have been shown to interact with the ERα promoter [109]. Interestingly, MeCP2 is also essential for normal brain development and plays a role in normal neuronal function [110]. It is present in high levels in mature neurons [111] and is thought to play a role in regulating genes that regulate synaptic function [112].
Methylation of the ERα promoter is also associated with atherosclerosis in humans [113]. Atherosclerotic plaques and aortic smooth muscle cells from patients with atherosclerosis have increased methylation of the ERα promoter as compared to controls. Additionally, the methylation of the ERα gene increases with age in cells of the right atrium. Thus, aberrant methylation of the ERα gene also appears to correlate with cardiovascular disease suggesting a global mechanism by which the ERα gene is regulated. An understanding of how the ERα gene is regulated in normal conditions is critical to understanding the aberrant regulation in pathological conditions.
Maternal Behavior
Maternal care of rodent pups can lead to long-term effects affecting the life-long response to stress in the offspring [114]. Mothers that have high rates of licking and grooming behavior have offspring with a more modest response to stress. Variations in the rates of licking and grooming are inherited such that mothers with high rates of licking and grooming activity have pups that show the same behavior when they become mothers [115]. These differences in maternal care have been linked to oxytocin levels in the medial preoptic area of the hypothalamus, and ovariectomy and E2 replacement can regulate this oxytocin-binding [116]. In rats of low licking and grooming mothers, however, E2 is not able to regulate oxytocin activity, suggesting a permanent alteration of estrogen receptor expression. Adult offspring of mothers that exhibited high licking and grooming activity had increased expression of ERα mRNA in the medial preoptic area of the hypothalamus, and this increased expression was associated with less methylation at the ERα 0/B promoter, while the ERα promoter in offspring from low licking and grooming mothers was hypermethylated [85]. Such long-term changes in expression due to DNA methylation suggest DNA methylation is a critical component of developmental gene expression. These studies are reviewed in-depth by Champagne in the present issue.
Development
As discussed above, ERα mRNA expression is dynamically regulated during development, particularly in the cortex. Recently, we have described the progressive methylation of two of the ERα promoters that are expressed in the neonatal mouse cortex [117]. Both Exon A and Exon C of the mouse ERα gene become methylated at postnatal day 10. This age corresponds with the beginning of the decline in ERα mRNA expression in the cortex. Furthermore, MeCP2 binds to these promoters at the same time they become methylated, further suggesting that methylation may play a role in the suppression of ERα mRNA in the developing brain and that ERα is a target of MeCP2 activity. Currently we are investigating the developmental regulation of these and other factors to determine their role in the regulation of ERα mRNA and the potential consequences of aberrant ERα mRNA expression.
Stroke
As discussed above, ERα mRNA is expressed at very low, nearly undetectable levels in the adult rodent cortex, but following a neuronal insult such as that initiated during a stroke, ERα is dramatically and rapidly reactivated [71; 118]. We have recently observed that ERα promoters are methylated in the female adult cortex when ERα mRNA expression is low [72]. The methylation status of the ERα promoter in male and intact female rats following MCAO was also examined (Figure 5). Genomic DNA was isolated from the cortex 24 hours after injury, and subjected to methylation-specific PCR (MSP). Using MSP targeting CpG islands within the promoter 0/B of the rat ERα gene, we found that ischemia decreased methylation in the ischemic cortex of females, while there was no change in methylation in the male. Furthermore, using chromatin immunoprecipitation assays, we examined the association of MeCP2 with the ERα promoter. MeCP2 was associated with the ERα promoter on the uninjured side of the brain of both male and female rats, but dissociated from the ERα promoter following injury only in female rats, corresponding with the methylation status of the promoter (Figure 6). These data are the first to demonstrate a difference in the regulation of ERα mRNA expression in response to MCAO between male and female rats and correlate DNA methylation with this sex difference. Future studies will determine if this sex difference plays a role in the ability of E2 to act as a neuroprotective factor.
Figure 5.
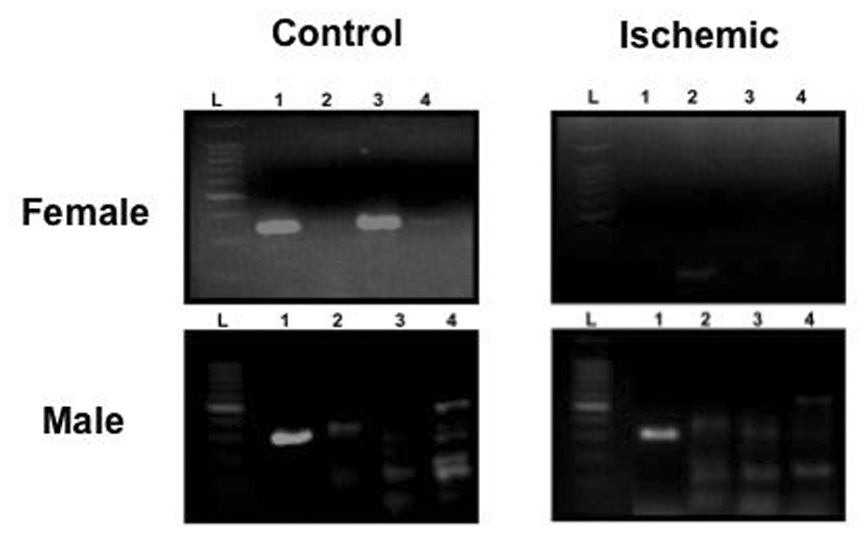
Ischemia changes the methylation status of the ERα promoter in female (Top), but not male (Bottom) rats. Gonadally intact animals underwent unilateral middle cerebral artery occlusion (MCAO) and tissue was harvested and frozen at −80°C twenty-four hours later. 250µM thick sections were taken on a cryostat and genomic DNA was extracted from micropunches from the ipsilateral (ischemic) and contralateral (control) sides of the cortex as previously described [72]. Genomic DNA was treated with sodium bisulfite to convert the unmethylated cytosines to uracils. Methylation-specific PCR on the bisulfite-modified DNA was performed using primers specific for methylated DNA for four different CpG islands (Lanes 1–4) within the rat ERα 0/B promoter. A 100 kb ladder (L) is pictured as a reference for size of the PCR products. Each experiment was repeated at least three separate times.
Figure 6.
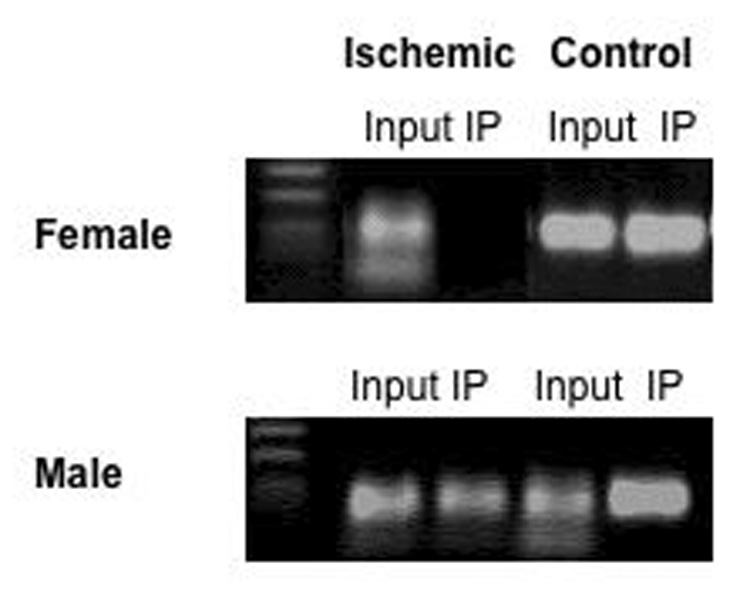
Chromatin immunoprecipitation assay (ChIP) using a MeCP2 antibody on nuclear extracts from both female (Top) and male (Bottom) rats was performed as previously described [139]. Genomic DNA was isolated from micropunches from the ipsilateral (ischemic) and contralateral (control) sides of the cortex twenty-four hours after middle cerebral artery occlusion. Protein-DNA complexes were cross-linked and sheared by sonication to produce 300 to 500 base pair fragments. These protein/DNA fragments were incubated with an antibody specific for MeCP2. The immunoprecipitated complexes were dissociated and the associated DNA was amplified by PCR with primers specific for the ERα 0/B promoter. PCR products were analyzed by agarose gel electrophoresis and visualized by ethidium bromide staining. Negative controls with non-specific IgGs were also performed. Input= sample before immunoprecipitation, IP= ChIP product after incubation with MeCP2 antibody. A 100 kb ladder is pictured as a reference for the size of the PCR products.
Not all changes in ERα mRNA expression, however, have been shown to correspond with methylation of the ERα promoter. Neonatal exposure to estrogenic compounds alters ERα mRNA in the mouse [36]. Rats exposed to the xenoestrogen, bisphenol A, during the first week of life had elevated ERα mRNA levels in the medial preoptic area of the hypothalamus at 21 days of age as compared to adults [36]. However, in this case, no changes in the methylation status of the ERα promoters examined were observed. Instead, bisphenol A altered the expression promoter usage suggesting a complex interaction between both promoter usage and promoter methylation. This suggests that both may be intimately related and that specific factors or stimuli can regulate one or both functions.
Potential Relationships with Human Neurological Function
Learning and Memory
Epigenetic regulation of gene expression in neurons is associated with long-term memory formation and synaptic plasticity [119; 120]. In the rodent hippocampus fear conditioning has been shown to induce DNA methyltransferase 3A (Dnmt3A) and Dnmt3B expression [121]. Additionally, fear conditioning in rats resulted in the rapid methylation of the protein phosphatase 1 gene promoter, a gene known to be involved with LTP and memory formation [119]. E2 has long been thought to influence specific types of learning and memory [122]. In rats, E2 has been shown to affect the development of LTP after fear conditioning [123; 124; 125]. Although the role of the classical ERα in mediating these effects is not clear, it remains possible, and likely, that membrane or cytoplasmic estrogen receptors mediate the effects of E2 on modulation of learning and memory.
Neurological Disorders
In recent years, alterations in DNA methylation have been observed to be associated with a variety of neurological disorders, including Rett Syndrome and schizophrenia [126; 127]. Mutations in the methyl-CpG-binding protein gene, MeCP2, are believed to be the cause of some cases of Rett syndrome [126]. Since MeCP2 is a central player in epigenetic regulation of gene expression, these data demonstrate the critical role of coordinated expression of genes during neural development and the potential critical role of regulating neuronal genes by epigenetic mechanisms. Recently, increasing evidence has emerged that epigenetic mechanisms are also involved in the development of schizophrenia [128; 129]. Taken together, these studies demonstrate an increased understanding of the importance of DNA methylation on the regulation and gene expression in neurons and as a potential therapeutic target for many neurological disorders.
A direct connection between E2 and estrogen receptor expression to these disorders has not been made, however E2 is known to influence many of these processes. E2 is implicated in modulating symptoms of major mental illnesses including depression and schizophrenia [130; 131; 132]. Symptom severity has been shown to fluctuate across the menstrual cycle and is often modified during pregnancy. Interestingly, although alterations in circulating E2 levels have not been consistently reported, recent studies do suggest a link between decreased E2 and schizophrenia in both men and women [133; 134; 135; 136; 137]. Additionally, ERα is expressed in the amygdala, hippocampus and cortex, brain regions thought to be involved in the development of schizophrenia, and a decline in ERα mRNA expression has been reported in the hippocampus of an individual with schizophrenia [138]. Although the connection between DNA methylation of the estrogen receptor and the development of neurological disorders has not been directly established, the potential exists that the mechanisms described in this review may play a role in estrogen receptor expression in the human brain. This possibility opens the door to future investigations integrating the role of epigenetic control of gene expression and the neuroendocrine brain.
Summary
Estrogens mediate numerous effects on the brain, and most require the presence of the estrogen receptor. Regulation of the ERα gene is critical for mediating these responses in an age, gender, and brain region-specific manner. Alterations in this regulation either during development, disease or aging could potentially interfere with estrogen action. We propose that numerous physiological influences can potentially regulate ERα gene expression using reversible epigenetic mechanisms. An understanding of these mechanisms could lead to our understanding of potential future therapeutic targets in disorders that require estrogen signaling. Investigation into this relatively unexplored mechanism of gene expression in the neuroendocrine brain is a rapidly growing area of research.
Acknowledgements
This work cited from our laboratory was supported by a COBRE grant P20 RR15592 from the National Center for Research Resources (NCRR), R01 HL073693 (MEW) and American Heart Association Predoctoral Fellowship 0615231B (AKP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88:91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211:1294–1302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- 3.Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci. 2006;9:220–226. doi: 10.1038/nn1624. [DOI] [PubMed] [Google Scholar]
- 4.Anderson RH, Fleming DE, Rhees RW, Kinghorn E. Relationships between sexual activity, plasma testosterone, and the volume of the sexually dimorphic nucleus of the preoptic area in prenatally stressed and non-stressed rats. Brain Res. 1986;370:1–10. doi: 10.1016/0006-8993(86)91098-x. [DOI] [PubMed] [Google Scholar]
- 5.Toran-Allerand CD. Sex steroids and the development of the newborn mouse hypothalamus and preoptic area in vitro: implications for sexual differentiation. Brain Res. 1976;106:407–412. doi: 10.1016/0006-8993(76)91038-6. [DOI] [PubMed] [Google Scholar]
- 6.Rhees RW, Shryne JE, Gorski RA. Onset of the hormone-sensitive perinatal period for sexual differentiation of the sexually dimorphic nucleus of the preoptic area in female rats. J Neurobiol. 1990;21:781–786. doi: 10.1002/neu.480210511. [DOI] [PubMed] [Google Scholar]
- 7.McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- 8.Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, Magarinos AM, Allen PB, Greengard P, Luine V, McEwen BS. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci U S A. 2004;101:2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- 10.Sherwin BB. Estrogenic effects on memory in women. Ann N Y Acad Sci. 1994;743:213–230. doi: 10.1111/j.1749-6632.1994.tb55794.x. discussion 230-1. [DOI] [PubMed] [Google Scholar]
- 11.Simpkins JW, Singh M, Bishop J. The potential role for estrogen replacement therapy in the treatment of the cognitive decline and neurodegeneration associated with Alzheimer's disease. Neurobiol Aging. 1994;15 Suppl 2:S195–S197. doi: 10.1016/0197-4580(94)90205-4. [DOI] [PubMed] [Google Scholar]
- 12.Wise PM, Dubal DB, Wilson ME, Rau SW, Liu Y. Estrogens: trophic and protective factors in the adult brain. Front Neuroendocrinol. 2001;22:33–66. doi: 10.1006/frne.2000.0207. [DOI] [PubMed] [Google Scholar]
- 13.Shughrue PJ, Stumpf WE, MacLusky NJ, Zielinski JE, Hochberg RB. Developmental changes in estrogen receptors in mouse cerebral cortex between birth and postweaning: studied by autoradiography with 11 beta-methoxy-16 alpha- [125I]iodoestradiol. Endocrinology. 1990;126:1112–1124. doi: 10.1210/endo-126-2-1112. [DOI] [PubMed] [Google Scholar]
- 14.Gerlach JL, McEwen BS, Toran-Allerand CD, Friedman WJ. Perinatal development of estrogen receptors in mouse brain assessed by radioautography, nuclear isolation and receptor assay. Brain Res. 1983;313:7–18. doi: 10.1016/0165-3806(83)90197-9. [DOI] [PubMed] [Google Scholar]
- 15.Toran-Allerand CD, Miranda RC, Hochberg RB, MacLusky NJ. Cellular variations in estrogen receptor mRNA translation in the developing brain: evidence from combined [125I]estrogen autoradiography and non-isotopic in situ hybridization histochemistry. Brain Res. 1992;576:25–41. doi: 10.1016/0006-8993(92)90606-a. [DOI] [PubMed] [Google Scholar]
- 16.Koike S, Sakai M, Muramatsu M. Molecular cloning and characterization of rat estrogen receptor cDNA. Nucleic Acids Res. 1987;15:2499–2513. doi: 10.1093/nar/15.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green S, Walter P, Kumar V, Krust A, Bornert JM, Argos P, Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986;320:134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- 18.White R, Lees JA, Needham M, Ham J, Parker M. Structural organization and expression of the mouse estrogen receptor. Mol Endocrinol. 1987;1:735–744. doi: 10.1210/mend-1-10-735. [DOI] [PubMed] [Google Scholar]
- 19.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mosselman S, Polman J, Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- 21.Xu Y, Traystman RJ, Hurn PD, Wang MM. Membrane restraint of estrogen receptor alpha enhances estrogen-dependent nuclear localization and genomic function. Mol Endocrinol. 2004;18:86–96. doi: 10.1210/me.2003-0262. [DOI] [PubMed] [Google Scholar]
- 22.Razandi M, Oh P, Pedram A, Schnitzer J, Levin ER. ERs associate with and regulate the production of caveolin: implications for signaling and cellular actions. Mol Endocrinol. 2002;16:100–115. doi: 10.1210/mend.16.1.0757. [DOI] [PubMed] [Google Scholar]
- 23.Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES, Jr, Nethrapalli IS, Tinnikov AA. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 25.Donahue JE, Stopa EG, Chorsky RL, King JC, Schipper HM, Tobet SA, Blaustein JD, Reichlin S. Cells containing immunoreactive estrogen receptor-alpha in the human basal forebrain. Brain Res. 2000;856:142–151. doi: 10.1016/s0006-8993(99)02413-0. [DOI] [PubMed] [Google Scholar]
- 26.Chaban VV, Lakhter AJ, Micevych P. A membrane estrogen receptor mediates intracellular calcium release in astrocytes. Endocrinology. 2004;145:3788–3795. doi: 10.1210/en.2004-0149. [DOI] [PubMed] [Google Scholar]
- 27.Osterlund MK, Grandien K, Keller E, Hurd YL. The human brain has distinct regional expression patterns of estrogen receptor alpha mRNA isoforms derived from alternative promoters. J Neurochem. 2000;75:1390–1397. doi: 10.1046/j.1471-4159.2000.0751390.x. [DOI] [PubMed] [Google Scholar]
- 28.Osterlund MK, Gustafsson JA, Keller E, Hurd YL. Estrogen receptor beta (ERbeta) messenger ribonucleic acid (mRNA) expression within the human forebrain: distinct distribution pattern to ERalpha mRNA. J Clin Endocrinol Metab. 2000;85:3840–3846. doi: 10.1210/jcem.85.10.6913. [DOI] [PubMed] [Google Scholar]
- 29.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez M, Cabrera-Socorro A, Perez-Garcia CG, Fraser JD, Lopez FJ, Alonso R, Meyer G. Distribution patterns of estrogen receptor alpha and beta in the human cortex and hippocampus during development and adulthood. J Comp Neurol. 2007;503:790–802. doi: 10.1002/cne.21419. [DOI] [PubMed] [Google Scholar]
- 31.Shughrue PJ, Merchenthaler I. Distribution of estrogen receptor beta immunoreactivity in the rat central nervous system. J Comp Neurol. 2001;436:64–81. [PubMed] [Google Scholar]
- 32.Perez SE, Chen EY, Mufson EJ. Distribution of estrogen receptor alpha and beta immunoreactive profiles in the postnatal rat brain. Brain Res Dev Brain Res. 2003;145:117–139. doi: 10.1016/s0165-3806(03)00223-2. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Y, Shughrue PJ, Dorsa DM. Estrogen receptor protein is differentially regulated in the preoptic area of the brain and in the uterus during the rat estrous cycle. Neuroendocrinology. 1995;61:276–283. doi: 10.1159/000126849. [DOI] [PubMed] [Google Scholar]
- 34.Pasterkamp RJ, Yuri K, Morita N, Kawata M. Differential expression of estrogen receptor mRNA and protein in the female rat preoptic area. Neurosci Lett. 1997;239:81–84. doi: 10.1016/s0304-3940(97)00888-4. [DOI] [PubMed] [Google Scholar]
- 35.Kos M, Reid G, Denger S, Gannon F. Minireview: genomic organization of the human ERalpha gene promoter region. Mol Endocrinol. 2001;15:2057–2063. doi: 10.1210/mend.15.12.0731. [DOI] [PubMed] [Google Scholar]
- 36.Monje L, Varayoud J, Luque EH, Ramos JG. Neonatal exposure to bisphenol A modifies the abundance of estrogen receptor {alpha} transcripts with alternative 5'-untranslated regions in the female rat preoptic area. J Endocrinol. 2007;194:201–212. doi: 10.1677/JOE-07-0014. [DOI] [PubMed] [Google Scholar]
- 37.Weigel RJ, Crooks DL, Iglehart JD, deConinck EC. Quantitative analysis of the transcriptional start sites of estrogen receptor in breast carcinoma. Cell Growth Differ. 1995;6:707–711. [PubMed] [Google Scholar]
- 38.Grandien K, Backdahl M, Ljunggren O, Gustafsson JA, Berkenstam A. Estrogen target tissue determines alternative promoter utilization of the human estrogen receptor gene in osteoblasts and tumor cell lines. Endocrinology. 1995;136:2223–2229. doi: 10.1210/endo.136.5.7720671. [DOI] [PubMed] [Google Scholar]
- 39.Grandien KF, Berkenstam A, Nilsson S, Gustafsson JA. Localization of DNase I hypersensitive sites in the human oestrogen receptor gene correlates with the transcriptional activity of two differentially used promoters. J Mol Endocrinol. 1993;10:269–277. doi: 10.1677/jme.0.0100269. [DOI] [PubMed] [Google Scholar]
- 40.Flouriot G, Griffin C, Kenealy M, Sonntag-Buck V, Gannon F. Differentially expressed messenger RNA isoforms of the human estrogen receptor-alpha gene are generated by alternative splicing and promoter usage. Mol Endocrinol. 1998;12:1939–1954. doi: 10.1210/mend.12.12.0209. [DOI] [PubMed] [Google Scholar]
- 41.Grandien K. Determination of transcription start sites in the human estrogen receptor gene and identification of a novel, tissue-specific, estrogen receptor-mRNA isoform. Mol Cell Endocrinol. 1996;116:207–212. doi: 10.1016/0303-7207(95)03716-0. [DOI] [PubMed] [Google Scholar]
- 42.Penolazzi L, Lambertini E, Giordano S, Sollazzo V, Traina G, del Senno L, Piva R. Methylation analysis of the promoter F of estrogen receptor alpha gene: effects on the level of transcription on human osteoblastic cells. J Steroid Biochem Mol Biol. 2004;91:1–9. doi: 10.1016/j.jsbmb.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 43.Hirata S, Koh T, Yamada-Mouri N, Hoshi K, Kato J. The untranslated first exon 'exon 0S' of the rat estrogen receptor (ER) gene. FEBS Lett. 1996;394:371–373. doi: 10.1016/0014-5793(96)00987-8. [DOI] [PubMed] [Google Scholar]
- 44.Hirata S, Koh T, Yamada-Mouri N, Kato J. The novel untranslated first exon "exon 0N" of the rat estrogen receptor gene. Biochem Biophys Res Commun. 1996;225:849–854. doi: 10.1006/bbrc.1996.1262. [DOI] [PubMed] [Google Scholar]
- 45.Freyschuss B, Grandien K. The 5' flank of the rat estrogen receptor gene: structural characterization and evidence for tissue- and species-specific promoter utilization. J Mol Endocrinol. 1996;17:197–206. doi: 10.1677/jme.0.0170197. [DOI] [PubMed] [Google Scholar]
- 46.Hamada T, Wada-Kiyama Y, Sakuma Y. Visualizing forebrain-specific usage of an estrogen receptor alpha promoter for receptor downregulation in the rat. Brain Res Mol Brain Res. 2005;139:42–51. doi: 10.1016/j.molbrainres.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 47.Kato J, Hirata S, Koh T, Yamada-Mouri N, Hoshi K, Okinaga S. The multiple untranslated first exons and promoters system of the oestrogen receptor gene in the brain and peripheral tissues of the rat and monkey and the developing rat cerebral cortex. J Steroid Biochem Mol Biol. 1998;65:281–293. doi: 10.1016/s0960-0760(97)00184-2. [DOI] [PubMed] [Google Scholar]
- 48.Osada N, Hirata S, Shoda T, Hoshi K. The novel untranslated exon "exon 0T" encoded between the exon 0 and exon 1 of the rat estrogen receptor alpha (ER alpha) gene. Endocr J. 2001;48:465–472. doi: 10.1507/endocrj.48.465. [DOI] [PubMed] [Google Scholar]
- 49.Kos M, O'Brien S, Flouriot G, Gannon F. Tissue-specific expression of multiple mRNA variants of the mouse estrogen receptor alpha gene. FEBS Lett. 2000;477:15–20. doi: 10.1016/s0014-5793(00)01750-6. [DOI] [PubMed] [Google Scholar]
- 50.Prewitt AK, Bisotti AJ, Trout AL, Jasper DK, Rosewell AN, Wilson ME. Developmental Regulation of Estrogen Receptor- alpha mRNA via Differential Promoter Usage in the Mouse Cortex. Endocrine Society Abstract. 2006 [Google Scholar]
- 51.Miranda RC, Toran-Allerand CD. Developmental expression of estrogen receptor mRNA in the rat cerebral cortex: a nonisotopic in situ hybridization histochemistry study. Cereb Cortex. 1992;2:1–15. doi: 10.1093/cercor/2.1.1. [DOI] [PubMed] [Google Scholar]
- 52.Prewitt AK, Wilson ME. Changes in estrogen receptor-alpha mRNA in the mouse cortex during development. Brain Res. 2007;1134:62–69. doi: 10.1016/j.brainres.2006.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 54.Pfaff D, Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J Comp Neurol. 1973;151:121–158. doi: 10.1002/cne.901510204. [DOI] [PubMed] [Google Scholar]
- 55.Sheridan PJ. Estrogen binding in the neonatal neocortex. Brain Res. 1979;178:201–206. doi: 10.1016/0006-8993(79)90101-x. [DOI] [PubMed] [Google Scholar]
- 56.O'Keefe JA, Li Y, Burgess LH, Handa RJ. Estrogen receptor mRNA alterations in the developing rat hippocampus. Brain Res Mol Brain Res. 1995;30:115–124. doi: 10.1016/0169-328x(94)00284-l. [DOI] [PubMed] [Google Scholar]
- 57.Paden CM, Gerlach JL, McEwen BS. Estrogen and progestin receptors appear in transplanted fetal hypothalamus-preoptic area independently of the steroid environment. J Neurosci. 1985;5:2374–2381. doi: 10.1523/JNEUROSCI.05-09-02374.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O'Keefe JA, Pedersen EB, Castro AJ, Handa RJ. The ontogeny of estrogen receptors in heterochronic hippocampal and neocortical transplants demonstrates an intrinsic developmental program. Brain Res Dev Brain Res. 1993;75:105–112. doi: 10.1016/0165-3806(93)90069-m. [DOI] [PubMed] [Google Scholar]
- 59.Shughrue PJ, Bushnell CD, Dorsa DM. Estrogen receptor messenger ribonucleic acid in female rat brain during the estrous cycle: a comparison with ovariectomized females and intact males. Endocrinology. 1992;131:381–388. doi: 10.1210/endo.131.1.1612018. [DOI] [PubMed] [Google Scholar]
- 60.Simerly RB, Young BJ. Regulation of estrogen receptor messenger ribonucleic acid in rat hypothalamus by sex steroid hormones. Mol Endocrinol. 1991;5:424–432. doi: 10.1210/mend-5-3-424. [DOI] [PubMed] [Google Scholar]
- 61.Kruijver FP, Balesar R, Espila AM, Unmehopa UA, Swaab DF. Estrogen receptor-alpha distribution in the human hypothalamus in relation to sex and endocrine status. J Comp Neurol. 2002;454:115–139. doi: 10.1002/cne.10416. [DOI] [PubMed] [Google Scholar]
- 62.Kruijver FP, Swaab DF. Sex hormone receptors are present in the human suprachiasmatic nucleus. Neuroendocrinology. 2002;75:296–305. doi: 10.1159/000057339. [DOI] [PubMed] [Google Scholar]
- 63.Dubal DB, Kashon ML, Pettigrew LC, Ren JM, Finklestein SP, Rau SW, Wise PM. Estradiol protects against ischemic injury. J Cereb Blood Flow Metab. 1998;18:1253–1258. doi: 10.1097/00004647-199811000-00012. [DOI] [PubMed] [Google Scholar]
- 64.Simpkins JW, Rajakumar G, Zhang YQ, Simpkins CE, Greenwald D, Yu CJ, Bodor N, Day AL. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J Neurosurg. 1997;87:724–730. doi: 10.3171/jns.1997.87.5.0724. [DOI] [PubMed] [Google Scholar]
- 65.Rusa R, Alkayed NJ, Crain BJ, Traystman RJ, Kimes AS, London ED, Klaus JA, Hurn PD. 17beta-estradiol reduces stroke injury in estrogen-deficient female animals. Stroke. 1999;30:1665–1670. doi: 10.1161/01.str.30.8.1665. [DOI] [PubMed] [Google Scholar]
- 66.Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–165. doi: 10.1161/01.str.29.1.159. discussion 166. [DOI] [PubMed] [Google Scholar]
- 67.Toung TJ, Traystman RJ, Hurn PD. Estrogen-mediated neuroprotection after experimental stroke in male rats. Stroke. 1998;29:1666–1670. doi: 10.1161/01.str.29.8.1666. [DOI] [PubMed] [Google Scholar]
- 68.Liu D, Smith CL, Barone FC, Ellison JA, Lysko PG, Li K, Simpson IA. Astrocytic demise precedes delayed neuronal death in focal ischemic rat brain. Brain Res Mol Brain Res. 1999;68:29–41. doi: 10.1016/s0169-328x(99)00063-7. [DOI] [PubMed] [Google Scholar]
- 69.Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci U S A. 2001;98:1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rau SW, Dubal DB, Wise PM. Estradiol protects against apoptotic cell death in stroke injury: possible mechanisms. Society for Neuroscience Abstracts. 2001;27:1165. [Google Scholar]
- 71.Dubal DB, Rau SW, Shughrue PJ, Zhu H, Yu J, Cashion AB, Suzuki S, Gerhold LM, Bottner MB, Dubal SB, Merchanthaler I, Kindy MS, Wise PM. Differential modulation of estrogen receptors (ERs) in ischemic brain injury: a role for ERalpha in estradiol-mediated protection against delayed cell death. Endocrinology. 2006;147:3076–3084. doi: 10.1210/en.2005-1177. [DOI] [PubMed] [Google Scholar]
- 72.Westberry JM, Prewitt AK, Wilson ME. Epigenetic Regulation of the Estrogen Receptor Alpha Promoter in the Cereberal Cortex Following Ischemia in Male and Female Rats. Neuroscience. 2008 doi: 10.1016/j.neuroscience.2008.01.048. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wilson ME, Dubal DB, Wise PM. Estradiol protects against injury-induced cell death in cortical explant cultures: a role for estrogen receptors. Brain Res. 2000;873:235–242. doi: 10.1016/s0006-8993(00)02479-3. [DOI] [PubMed] [Google Scholar]
- 74.Wilson ME, Liu Y, Wise PM. Estradiol enhances Akt activation in cortical explant cultures following neuronal injury. Molecular Brain Research. 2002;102:48–54. doi: 10.1016/s0169-328x(02)00181-x. [DOI] [PubMed] [Google Scholar]
- 75.Prewitt AK, Vula F, Westberry JM, Wilson ME. Estrogen Receptor -alpha mRNA Regulation in the Mouse Cortex via Methylation Following Injury In Vitro. Society for Neuroscience Abstract. 2007 [Google Scholar]
- 76.Pinzone JJ, Stevenson H, Strobl JS, Berg PE. Molecular and cellular determinants of estrogen receptor alpha expression. Mol Cell Biol. 2004;24:4605–4612. doi: 10.1128/MCB.24.11.4605-4612.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stoica A, Pentecost E, Martin MB. Effects of arsenite on estrogen receptor-alpha expression and activity in MCF-7 breast cancer cells. Endocrinology. 2000;141:3595–3602. doi: 10.1210/endo.141.10.7704. [DOI] [PubMed] [Google Scholar]
- 78.Stoica A, Saceda M, Fakhro A, Joyner M, Martin MB. Role of insulin-like growth factor-I in regulating estrogen receptor-alpha gene expression. J Cell Biochem. 2000;76:605–614. doi: 10.1002/(sici)1097-4644(20000315)76:4<605::aid-jcb9>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 79.Swami S, Krishnan AV, Feldman D. 1alpha,25-Dihydroxyvitamin D3 down-regulates estrogen receptor abundance and suppresses estrogen actions in MCF-7 human breast cancer cells. Clin Cancer Res. 2000;6:3371–3379. [PubMed] [Google Scholar]
- 80.Savoldi G, Ferrari F, Ruggeri G, Sobek L, Albertini A, Di Lorenzo D. Progesterone agonists and antagonists induce down- and up-regulation of estrogen receptors and estrogen inducible genes in human breast cancer cell lines. Int J Biol Markers. 1995;10:47–54. doi: 10.1177/172460089501000109. [DOI] [PubMed] [Google Scholar]
- 81.Stoica A, Saceda M, Doraiswamy VL, Coleman C, Martin MB. Regulation of estrogen receptor-alpha gene expression by epidermal growth factor. J Endocrinol. 2000;165:371–378. doi: 10.1677/joe.0.1650371. [DOI] [PubMed] [Google Scholar]
- 82.Chiang CH, Cheng KW, Igarashi S, Nathwani PS, Leung PC. Hormonal regulation of estrogen receptor alpha and beta gene expression in human granulosa-luteal cells in vitro. J Clin Endocrinol Metab. 2000;85:3828–3839. doi: 10.1210/jcem.85.10.6886. [DOI] [PubMed] [Google Scholar]
- 83.Frasor J, Gibori G. Prolactin regulation of estrogen receptor expression. Trends Endocrinol Metab. 2003;14:118–123. doi: 10.1016/s1043-2760(03)00030-4. [DOI] [PubMed] [Google Scholar]
- 84.Frasor J, Barkai U, Zhong L, Fazleabas AT, Gibori G. PRL-induced ERalpha gene expression is mediated by Janus kinase 2 (Jak2) while signal transducer and activator of transcription 5b (Stat5b) phosphorylation involves Jak2 and a second tyrosine kinase. Mol Endocrinol. 2001;15:1941–1952. doi: 10.1210/mend.15.11.0722. [DOI] [PubMed] [Google Scholar]
- 85.Champagne FA, Weaver IC, Diorio J, Dymov S, Szyf M, Meaney MJ. Maternal care associated with methylation of the estrogen receptor-alpha 1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology. 2006;147:2909–2915. doi: 10.1210/en.2005-1119. [DOI] [PubMed] [Google Scholar]
- 86.Wolffe AP, Matzke MA. Epigenetics: regulation through repression. Science. 1999;286:481–486. doi: 10.1126/science.286.5439.481. [DOI] [PubMed] [Google Scholar]
- 87.Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci. 2006;31:89–97. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 88.Rakyan VK, Preis J, Morgan HD, Whitelaw E. The marks, mechanisms and memory of epigenetic states in mammals. Biochem J. 2001;356:1–10. doi: 10.1042/0264-6021:3560001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cooper DN, Krawczak M. Cytosine methylation and the fate of CpG dinucleotides in vertebrate genomes. Hum Genet. 1989;83:181–188. doi: 10.1007/BF00286715. [DOI] [PubMed] [Google Scholar]
- 90.Bird AP, Wolffe AP. Methylation-induced repression--belts, braces, and chromatin. Cell. 1999;99:451–454. doi: 10.1016/s0092-8674(00)81532-9. [DOI] [PubMed] [Google Scholar]
- 91.Kiefer JC. Epigenetics in development. Dev Dyn. 2007;236:1144–1156. doi: 10.1002/dvdy.21094. [DOI] [PubMed] [Google Scholar]
- 92.Levenson JM, Sweatt JD. Epigenetic mechanisms in memory formation. Nat Rev Neurosci. 2005;6:108–118. doi: 10.1038/nrn1604. [DOI] [PubMed] [Google Scholar]
- 93.Ng HH, Zhang Y, Hendrich B, Johnson CA, Turner BM, Erdjument-Bromage H, Tempst P, Reinberg D, Bird A. MBD2 is a transcriptional repressor belonging to the MeCP1 histone deacetylase complex. Nat Genet. 1999;23:58–61. doi: 10.1038/12659. [DOI] [PubMed] [Google Scholar]
- 94.Ng HH, Jeppesen P, Bird A. Active repression of methylated genes by the chromosomal protein MBD1. Mol Cell Biol. 2000;20:1394–1406. doi: 10.1128/mcb.20.4.1394-1406.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, Bird A. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- 96.Ballestar E, Wolffe AP. Methyl-CpG-binding proteins. Targeting specific gene repression. Eur J Biochem. 2001;268:1–6. doi: 10.1046/j.1432-1327.2001.01869.x. [DOI] [PubMed] [Google Scholar]
- 97.Wade PA. Methyl CpG-binding proteins and transcriptional repression. Bioessays. 2001;23:1131–1137. doi: 10.1002/bies.10008. [DOI] [PubMed] [Google Scholar]
- 98.Issa JP, Ottaviano YL, Celano P, Hamilton SR, Davidson NE, Baylin SB. Methylation of the oestrogen receptor CpG island links ageing and neoplasia in human colon. Nat Genet. 1994;7:536–540. doi: 10.1038/ng0894-536. [DOI] [PubMed] [Google Scholar]
- 99.Issa JP, Zehnbauer BA, Civin CI, Collector MI, Sharkis SJ, Davidson NE, Kaufmann SH, Baylin SB. The estrogen receptor CpG island is methylated in most hematopoietic neoplasms. Cancer Res. 1996;56:973–977. [PubMed] [Google Scholar]
- 100.Oh AS, Lorant LA, Holloway JN, Miller DL, Kern FG, El-Ashry D. Hyperactivation of MAPK induces loss of ERalpha expression in breast cancer cells. Mol Endocrinol. 2001;15:1344–1359. doi: 10.1210/mend.15.8.0678. [DOI] [PubMed] [Google Scholar]
- 101.O'Doherty AM, Church SW, Russell SE, Nelson J, Hickey I. Methylation status of oestrogen receptor-alpha gene promoter sequences in human ovarian epithelial cell lines. Br J Cancer. 2002;86:282–284. doi: 10.1038/sj.bjc.6600028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ottaviano YL, Issa JP, Parl FF, Smith HS, Baylin SB, Davidson NE. Methylation of the estrogen receptor gene CpG island marks loss of estrogen receptor expression in human breast cancer cells. Cancer Res. 1994;54:2552–2555. [PubMed] [Google Scholar]
- 103.Sasaki M, Tanaka Y, Perinchery G, Dharia A, Kotcherguina I, Fujimoto S, Dahiya R. Methylation and inactivation of estrogen, progesterone, and androgen receptors in prostate cancer. J Natl Cancer Inst. 2002;94:384–390. doi: 10.1093/jnci/94.5.384. [DOI] [PubMed] [Google Scholar]
- 104.Lapidus RG, Nass SJ, Butash KA, Parl FF, Weitzman SA, Graff JG, Herman JG, Davidson NE. Mapping of ER gene CpG island methylation-specific polymerase chain reaction. Cancer Res. 1998;58:2515–2519. [PubMed] [Google Scholar]
- 105.Yang X, Phillips DL, Ferguson AT, Nelson WG, Herman JG, Davidson NE. Synergistic activation of functional estrogen receptor (ER)-alpha by DNA methyltransferase and histone deacetylase inhibition in human ER-alpha-negative breast cancer cells. Cancer Res. 2001;61:7025–7029. [PubMed] [Google Scholar]
- 106.Hortobagyi GN. Treatment of breast cancer. N Engl J Med. 1998;339:974–984. doi: 10.1056/NEJM199810013391407. [DOI] [PubMed] [Google Scholar]
- 107.Giacinti L, Claudio PP, Lopez M, Giordano A. Epigenetic information and estrogen receptor alpha expression in breast cancer. Oncologist. 2006;11:1–8. doi: 10.1634/theoncologist.11-1-1. [DOI] [PubMed] [Google Scholar]
- 108.Ferguson AT, Lapidus RG, Baylin SB, Davidson NE. Demethylation of the estrogen receptor gene in estrogen receptor-negative breast cancer cells can reactivate estrogen receptor gene expression. Cancer Res. 1995;55:2279–2283. [PubMed] [Google Scholar]
- 109.Sharma D, Blum J, Yang X, Beaulieu N, Macleod AR, Davidson NE. Release of methyl CpG binding proteins and histone deacetylase 1 from the Estrogen receptor alpha (ER) promoter upon reactivation in ER-negative human breast cancer cells. Mol Endocrinol. 2005;19:1740–1751. doi: 10.1210/me.2004-0011. [DOI] [PubMed] [Google Scholar]
- 110.Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet. 2001;27:322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- 112.Meehan RR, Lewis JD, Bird AP. Characterization of MeCP2, a vertebrate DNA binding protein with affinity for methylated DNA. Nucleic Acids Res. 1992;20:5085–5092. doi: 10.1093/nar/20.19.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang H, Chan SA, Ogier M, Hellard D, Wang Q, Smith C, Katz DM. Dysregulation of brain-derived neurotrophic factor expression and neurosecretory function in Mecp2 null mice. J Neurosci. 2006;26:10911–10915. doi: 10.1523/JNEUROSCI.1810-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Post WS, Goldschmidt-Clermont PJ, Wilhide CC, Heldman AW, Sussman MS, Ouyang P, Milliken EE, Issa JP. Methylation of the estrogen receptor gene is associated with aging and atherosclerosis in the cardiovascular system. Cardiovasc Res. 1999;43:985–991. doi: 10.1016/s0008-6363(99)00153-4. [DOI] [PubMed] [Google Scholar]
- 114.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 115.Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- 116.Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci U S A. 2001;98:12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Prewitt AK, Sengoku T, Vula F, Wilson ME. Developmental regulation of estrogen receptor-alpha mRNA via promoter methylation in the mouse cortex. Endocrine Society Abstract. 2007 [Google Scholar]
- 118.Dubal DB, Shughrue PJ, Wilson ME, Merchenthaler I, Wise PM. Estradiol modulates bcl-2 in cerebral ischemia: a potential role for estrogen receptors. J Neurosci. 1999;19:6385–6393. doi: 10.1523/JNEUROSCI.19-15-06385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Levenson JM, Roth TL, Lubin FD, Miller CA, Huang IC, Desai P, Malone LM, Sweatt JD. Evidence that DNA (cytosine-5) methyltransferase regulates synaptic plasticity in the hippocampus. J Biol Chem. 2006;281:15763–15773. doi: 10.1074/jbc.M511767200. [DOI] [PubMed] [Google Scholar]
- 120.Bailey CH, Kandel ER, Si K. The persistence of long-term memory: a molecular approach to self-sustaining changes in learning-induced synaptic growth. Neuron. 2004;44:49–57. doi: 10.1016/j.neuron.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 121.Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007;53:857–869. doi: 10.1016/j.neuron.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 122.Sherwin BB. Estrogen and cognitive functioning in women. Endocr Rev. 2003;24:133–151. doi: 10.1210/er.2001-0016. [DOI] [PubMed] [Google Scholar]
- 123.Ogiue-Ikeda M, Tanabe N, Mukai H, Hojo Y, Murakami G, Tsurugizawa T, Takata N, Kimoto T, Kawato S. Rapid modulation of synaptic plasticity by estrogens as well as endocrine disrupters in hippocampal neurons. Brain Res Rev. 2007 doi: 10.1016/j.brainresrev.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 124.Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 17beta-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- 125.Bi R, Broutman G, Foy MR, Thompson RF, Baudry M. The tyrosine kinase and mitogen-activated protein kinase pathways mediate multiple effects of estrogen in hippocampus. Proc Natl Acad Sci U S A. 2000;97:3602–3607. doi: 10.1073/pnas.060034497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Moretti P, Zoghbi HY. MeCP2 dysfunction in Rett syndrome and related disorders. Curr Opin Genet Dev. 2006;16:276–281. doi: 10.1016/j.gde.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 127.Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- 128.Tamminga CA, Holcomb HH. Phenotype of schizophrenia: a review and formulation. Mol Psychiatry. 2005;10:27–39. doi: 10.1038/sj.mp.4001563. [DOI] [PubMed] [Google Scholar]
- 129.Tremolizzo L, Carboni G, Ruzicka WB, Mitchell CP, Sugaya I, Tueting P, Sharma R, Grayson DR, Costa E, Guidotti A. An epigenetic mouse model for molecular and behavioral neuropathologies related to schizophrenia vulnerability. Proc Natl Acad Sci U S A. 2002;99:17095–17100. doi: 10.1073/pnas.262658999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gattaz WF, Vogel P. A. Riecher-Rossler, and G. Soddu, Influence of the menstrual cycle phase on the therapeutic response in schizophrenia. Biol Psychiatry. 1994;36:137–139. doi: 10.1016/0006-3223(94)91195-9. [DOI] [PubMed] [Google Scholar]
- 131.Freeman MP, Smith KW, Freeman SA, McElroy SL, Kmetz GE, Wright R, Keck PE., Jr The impact of reproductive events on the course of bipolar disorder in women. J Clin Psychiatry. 2002;63:284–287. doi: 10.4088/jcp.v63n0403. [DOI] [PubMed] [Google Scholar]
- 132.Chang SS, Renshaw DC. Psychosis and pregnancy. Compr Ther. 1986;12:36–41. [PubMed] [Google Scholar]
- 133.Baischer W, Koinig G, Hartmann B, Huber J, Langer G. Hypothalamic-pituitary-gonadal axis in depressed premenopausal women: elevated blood testosterone concentrations compared to normal controls. Psychoneuroendocrinology. 1995;20:553–559. doi: 10.1016/0306-4530(94)00081-k. [DOI] [PubMed] [Google Scholar]
- 134.Ozcan ME, Banoglu R. Gonadal hormones in schizophrenia and mood disorders. Eur Arch Psychiatry Clin Neurosci. 2003;253:193–196. doi: 10.1007/s00406-003-0424-7. [DOI] [PubMed] [Google Scholar]
- 135.Rubinow DR, Hoban MC, Grover GN, Galloway DS, Roy-Byrne P, Andersen R, Merriam GR. Changes in plasma hormones across the menstrual cycle in patients with menstrually related mood disorder and in control subjects. Am J Obstet Gynecol. 1988;158:5–11. doi: 10.1016/0002-9378(88)90765-x. [DOI] [PubMed] [Google Scholar]
- 136.Bergemann N, Mundt C, Parzer P, Jannakos I, Nagl I, Salbach B, Klinga K, Runnebaum B, Resch F. Plasma concentrations of estradiol in women suffering from schizophrenia treated with conventional versus atypical antipsychotics. Schizophr Res. 2005;73:357–366. doi: 10.1016/j.schres.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 137.Huber TJ, Tettenborn C, Leifke E, Emrich HM. Sex hormones in psychotic men. Psychoneuroendocrinology. 2005;30:111–114. doi: 10.1016/j.psyneuen.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 138.Perlman WR, Tomaskovic-Crook E, Montague DM, Webster MJ, Rubinow DR, Kleinman JE, Weickert CS. Alteration in estrogen receptor alpha mRNA levels in frontal cortex and hippocampus of patients with major mental illness. Biol Psychiatry. 2005;58:812–824. doi: 10.1016/j.biopsych.2005.04.047. [DOI] [PubMed] [Google Scholar]
- 139.Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, Jaenisch R, Greenberg ME. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302:885–889. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]


