Abstract
Cortactin is a cytoskeletal protein and src kinase substrate that is frequently overexpressed in cancer. Animal studies suggest that cortactin overexpression increases tumor aggressiveness, possibly through promotion of tumor invasion and metastasis. Recently, many studies have documented a role for cortactin in promoting cell motility and invasion, including a critical role in invadopodia, actin rich-subcellular protrusions associated with degradation of the extracellular matrix by cancer cells. Here, I review the evidence and potential mechanisms for cortactin as a critical mediator of tumor cell invasion.
Keywords: cortactin, tumor, invasion, migration, invadopodia
1. Introduction
Cortactin was first identified as one of the major substrates for src kinase [1]. Because it localized to cortical actin structures, it was named cortactin [2]. At that time, little was known about its function, except that it bound to actin filaments, had an SH3 domain, and was phosphorylated in its C-terminus by src kinase [2]. Subsequently, the cortactin gene was found to be identical with Ems1 [3], a gene that is frequently overexpressed in breast and head and neck cancers due to its presence in the 11q13 amplicon [4]. 11q13 amplification has been frequently tied to poor prognosis, including association with higher pathological stage, lymph node and distant metastasis, and decreased survival [5–13]. Although many other genes are present within this amplicon, the consistent overexpression of cortactin in 11q13-amplified tumors along with its ubiquitous presence in cell motility structures, such as lamellipodia and invadopodia [2,3], have generated a great deal of interest in the role of cortactin in tumor invasion.
2. Domain structure and binding partners
Cortactin has four major domains of interest: the N-terminal acidic (NTA) and tandem repeats domains, and the C-terminal proline-rich and SH3 domains (Fig 1). The N-terminus has generally been thought of as the actin-assembly region of the molecule, as binding sites for the Arp2/3 complex and for actin filaments are found in the NTA and repeats domains, respectively (Fig 1). These binding sites are both necessary and sufficient for direct regulation of Arp2/3- complex-mediated branched actin assembly (see below) [14,15]. In addition, localization of cortactin to sites of dynamic actin assembly in cells is frequently mediated through the Arp2/3 and F-actin-binding sites [14,16–18], suggesting that cortactin is both a sensor and regulator of branched actin assembly. The C-terminus is generally thought to be the regulatory or signaling end of the molecule, as the proline-rich domain contains phosphorylation sites for a number of kinases and the SH3 domain mediates binding to a variety of other signaling proteins (Table 1). However, many cytoskeletal and membrane trafficking proteins also bind to the SH3 domain of cortactin (Table 1), suggesting a potential scaffold or regulatory role for cortactin in cytoskeletal arrangement and membrane trafficking. Many of these cortactin binding partners, such as N-WASp, dynamin, and WIP, bind each other via separate direct interactions, suggesting that they may all function together in large multiprotein complexes.
Figure 1. Cortactin domain structure and binding partners.
Cortactin structure and domains is depicted in bright blue. Actin filaments (F-actin) is in light blue. Cortactin binding partners are indicated by labeling within the yellow boxes. The N-terminus of cortactin is composed of the N-terminal acidic (NTA) domain and the cortactin repeats (each repeat is represented by a numbered diamond) region. These two domains are notable for binding Arp2/3 complex and F-actin, respectively. The repeat is depicted binding to F-actin, because it is essential for that interaction; however, the other repeat regions contribute to binding affinity [18]. In the C-terminus, the proline rich region contains well-characterized serine and tyrosine phosphorylation sites. SS indicates the Erk S405 and S418 sites, whereas YYY indicates the Y421, Y466, and Y488 sites that are phosphorylated by src and the other tyrosine kinases indicated in the yellow box. Some of the cytoskeletal, membrane trafficking, and signaling proteins that bind to the SH3 domain are indicated in the yellow box below it. Biochemical activities regulated by the N-terminus and C-terminus are indicated at the bottom of the figure. For a full listing of binding partners, see Table 1. Illustration by Emily Clark, Ph.D.
Table 1.
Cortactin Binding Partners
| Protein | Cortactin Binding Domain | Function | Reference |
|---|---|---|---|
| Arp2/3 complex | NTA | Actin Assembly | [18] |
| Actin Filaments | Repeats | Actin Assembly | [2] |
| Caldesmon | N-terminus | Actin Assembly | [84] |
| Nck | phospho-Y421, 466, 482 | Adaptor Protein | [80] |
| Kinases | Unknown | Signal Transduction | |
| K+ Channel Kv1.2 | Unknown | Membrane excitability | [85] |
| BK Channels | SH3 domain | Membrane excitability | [86] |
| N-WASp | SH3 domain | Actin Assembly | [87] |
| WIP | SH3 domain | Actin Assembly | [88] |
| MIM | SH3 domain | Actin Assembly | [89] |
| Dynamin 2 | SH3 domain | Membrane Trafficking | [90] |
| AMAP1/ASAP1 | SH3 domain | Membrane Trafficking | |
| Signal Transduction | [91] | ||
| Hip1R | SH3 domain | Membrane Trafficking | [92] |
| ZO-1 | SH3 domain | Tight junction adaptor | [93] |
| MLCK | SH3 domain | Signal Transduction | [94] |
| CortBP1/SHANK2 | SH3 domain | Adaptor Protein | [95] |
| CD2AP | SH3 domain | Adaptor Protein | [96] |
| BPGAP1 | SH3 domain | Rho-GAP | [97] |
| Fgd1 | SH3 domain | Rho-GEF | [98] |
| CBP90 | SH3 domain | Unknown | [99] |
3. Cortactin as a regulator of branched actin assembly
The role of cortactin in actin assembly remained elusive for almost a decade after its discovery, when it was identified as a binding partner for the Arp2/3 complex through mass spectrometry analysis [18]. The Arp2/3 complex is the molecular machine that nucleates branched actin filament networks in cells [19]. Branched actin provides structural support for the plasma membrane and provides the force for such processes as protrusion of lamellipodia, vesicle trafficking, pathogen motility and formation of cell-cell junctions [19]. Although the critical and strong activators of the Arp2/3 complex are members of the Wiskott-Aldrich Syndrome family of proteins (WASps) [19,20], cortactin promotes weak activation of Arp2/3-mediated branched actin nucleation by enhancing the key step of association of the Arp2/3 complex with the side of mother actin filaments [14,15]. This activity may indirectly enhance WASp-induced nucleation at a variety of sites throughout the cell [20–22]. In addition, cortactin stabilizes branched actin networks after they are formed [15]. Inhibition of debranching appears to be unique to cortactin and is likely to be a major function of cortactin throughout the cell, since cortactin localizes to all cellular branched actin networks [15,23,24]. Regulation of debranching may be a critical step in processes that are dependent on branched actin, as recent work in budding yeast demonstrates that an Arp2/3 complex mutant that is defective in debranching has defects in endocytosis and actin network disassembly [25].
In addition to directly regulating actin assembly by the Arp2/3 complex through its N-terminus [14,15], several studies have demonstrated that cortactin can activate its binding partner N-WASp, by binding of the cortactin SH3 domain to the N-WASp proline-rich domain and relieving autoinhibition [26–28]. This effect is particularly strong in the presence of Nck, a tyrosine-kinase adaptor that binds to phosphorylated cortactin via an SH2 domain (Fig 1) and links to N-WASp through WIP [28]. In cells, the importance of these interactions may vary, as in some studies the SH3 domain and/or src kinase phosphorylation sites (that link to Nck) are critical for actin-based phenotypes [26,29,30], whereas in others only the N-terminus is required [16,31]. Thus, it is likely that cortactin may regulate actin assembly through multiple mechanisms, including stabilizing branches and enhancing Arp2/3-mediated nucleation through direct interactions of the N-terminus, as well through activation of N-WASp via interactions at the C-terminus.
4. Cortactin promotes cell migration
The strong localization of cortactin to cell motility structures such as lamellipodia and invadopodia [2,3] sparked an early interest in the role of cortactin in cell migration and invasion. Indeed, overexpression of cortactin has been shown to enhance cell motility in a variety of assays, including transwell migration [16,32], wound closure [26,33], and single cell motility [16]. Conversely, siRNA against cortactin inhibits cell motility in many of the same assays [16,34–36]. During Drosophila melanogaster oogenesis, the migration of border cells in egg chambers is impaired in cortactin-deficient flies, indicating that cortactin can regulate cell migration in vivo as well as in vitro [37].
Mechanistically, cortactin regulates the persistence [16], but not the ability to induce lamellipodial protrusions [16,30,36,38,39]. Cortactin also affects the ability of cells to form a dominant lamellipodium in response to growth factor stimulation [38]. In addition, our laboratory found that cortactin promotes the formation of new adhesions at the cell edge, which is likely to contribute to both the lamellipodial persistence and migration phenotypes [16]. The lamellipodial persistence, adhesion, and cell motility defects can be fully rescued by cortactin molecules that contain only the Arp2/3 complex and F-actin binding sites, suggesting that direct regulation of Arp2/3-mediated branched actin assembly and/or stability by cortactin is responsible [16]. However, other laboratories have reported a role for cortactin tyrosine phosphorylation [33,35], or SH3 domain interactions [26] in cell migration. Thus, cortactin may have different effects depending on the cellular context. This is not too surprising, since cortactin may affect actin assembly in different ways, e.g. via direct regulation of Arp2/3 complex by the N-terminus vs. activation of N-WASp through C-terminal interactions. In addition, cortactin has increasingly recognized cellular roles outside of the lamellipodia that might affect cell motility, such as in cell-cell adhesion [31,40,41] and membrane trafficking [42–46]. For example, regulation by cortactin of endocytosis of integrins and growth factor receptors [47] or secretion of molecules such as proteases [48] or extracellular matrix (ECM) could have major effects on cell motility that would be cell context dependent. Future studies should address these possibilities.
5. Cortactin in invasion: invadopodia and ECM degradation
In vivo, cell movement through tissues frequently requires the degradation of ECM. Cortactin appears to play a central role in this process, as it is important for invasion through matrix barriers, such as Matrigel- or gelatin-coated transwell filters [16,34–36]. At the subcellular level, cortactin is a critical component of invadopodia, actin-rich protrusions associated with ECM degradation [49].
Invadopodia were first identified in src kinase-transformed cells and are thought to constitute the invasive cellular machinery. They are characterized by the colocalization of many proteins that are found in focal adhesions and lamellipodia, as well as membrane trafficking proteins and proteases [49]. Related structures, podosomes, are found in osteoclasts, macrophages, and other normal cells that must cross tissue barriers or remodel ECM [49,50].
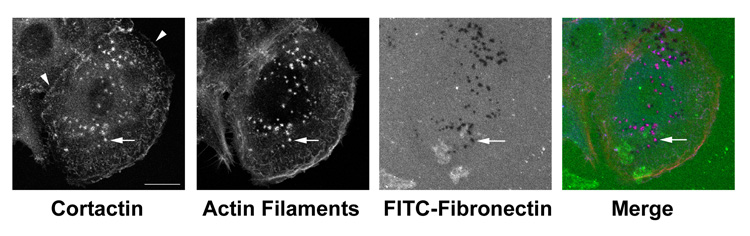
Cortactin is frequently used as an invadopodia marker, based on its strong localization to sites of focal ECM degradation (Fig 2). Studies in both podosomes and invadopodia indicate that cortactin is a universally important player in their function [30,48,51–54]. As in other areas of cortactin biology, there are multiple potential mechanisms for the strong effect of cortactin in this organelle. Cortactin may be acting directly to promote actin assembly at invadopodia puncta [55]. In osteoclasts, cortactin was found to be essential for the formation of actin-based podosomes, but not for other actin-based structures such as lamellipodia [30]. Similarly in src-transformed or overexpressing cells, cortactin downregulation reduces the number of actin-rich podosome rings [54] and invadopodia puncta [51]. Many key invadopodia proteins are also cortactin-binding partners, including N-WASp, WIP, dynamin, ASAP1/AMAP1, and src kinase [49], suggesting that cortactin may play a scaffolding function in invadopodia. Consistent with that idea, mutations in the cortactin C-terminal phosphorylation and binding sites have strong effects on invadopodia number and function [54,56].
Figure 2. Cortactin is a strong invadopodia marker.
Primary head and neck squamous carcinoma cells were isolated from tumors and plated, without previous culture, on coverslips coated with a thin film of crosslinked gelatin overlaid with FITC-Fibronectin, as previously described [48]. After 36 h, cells were fixed and immunostained with 4F11 mAb against cortactin, followed by an anti-mouse AlexaFluor-633 secondary antibody (Invitrogen) (blue in merge), and stained for actin filaments with rhodamine-phalloidin (red in merge). Degraded areas of ECM are evident as dark holes in the FITC-Fibronectin (green in merge) image. An arrow points to an example invadopodia in each of the separated and merged images. Note the colocalization of cortactin and actin punta with focal degradation of the ECM. Arrowheads in the cortactin image point out cortactin localization to other sites of branched actin assembly: lamellipodia at the edge of the cell and cell-cell junctions. Scale bar = 10 µm. Unpublished data by Emily Clark, Ph.D.
Regulation of membrane trafficking by cortactin is likely to be a critical component of invadopodia-associated ECM degradation. Recent live cell imaging studies from Susette Mueller’s laboratory demonstrate that cortactin recruitment precedes by a few minutes the trafficking of proteases to future sites of ECM degradation [51]. Interestingly, the presence of both cortactin and strong phosphotyrosine staining at invadopodia puncta was shown by the same group to identify a subset of invadopodia that are actively degrading ECM, suggesting that phosphotyrosine signaling and cortactin may be critical components of delivery and/or activation of invadopodia proteases [57]. Consistent with that idea, our recent data indicate that cortactin is essential for the trafficking of the key invadopodia proteases, MT1-MMP, MMP2, and MMP9 in head and neck squamous carcinoma cells (HNSCC) [48,58]. Although invadopodia-like actin puncta can still be formed in cortactin-KD cells, MMPs no longer localize to them and cell-associated ECM degradation does not occur [48,58]. At this point it is unclear whether cortactin function at the Golgi apparatus [43] or within invadopodial protrusions is more important for the delivery and targeting of proteases to invadopodia.
6. Cortactin function in tumors
Cortactin is overexpressed in many types of human cancers, including head and neck and esophageal squamous carcinomas, colorectal, gastric, hepatocellular, breast and ovarian cancers [9,11,59–61]. Most frequently cortactin overexpression occurs through chromosomal amplification of the 11q13.3 region [9], however, overexpression has also been reported in tumors without that amplification [61,62]. In HNSCC 30–40% of tumors contain the 11q13 amplicon and it clearly correlates with poor patient prognosis, including decreased survival [5–8,11–13]. In addition to cortactin, a number of interesting genes are present in this amplicon, including TPC2, ORAOV1/TAOS1, FGF4, CCND1, FGF19, FGF3, FLJ10261, FADD, PP1A1, TMEM1GA, and SHANK2 [63]. Of these, cyclinD1 and EMS1/cortactin have been considered to be the best candidates for promoting tumor aggressiveness, since unlike many of the other genes in the amplicon they are consistently overexpressed upon amplification [64]. Between these two genes, cortactin has been more highly correlated with poor prognosis in HNSCC and estrogen receptor-negative breast cancers, whereas cyclin D1 has been associated with poor prognosis in estrogen receptor-positive breast cancers [10,11,59,65]. However, since they are frequently overexpressed and amplified together they may function synergistically in many situations. Although there have been few studies to address the role of coexpressed 11q13 genes, Timpson et al. recently reported that cooverexpression of cyclinD1 and cortactin in HNSCC cells promotes resistance to gefitinib, an EGF receptor antagonist drug, as defined by IC50 for proliferation [66]. Rothschild et al. examined 11q13-amplified HNSCC cells and found that cortactin plays a generally important role in migration and invasion in this context and also promotes resistance to gefitinib-induced inhibition of migration; however it is unclear whether any of the other genes in the amplicon participate in this phenotype [35]. A recent study in HNSCC reports increased RNA expression of 6 additional genes within the 11q13.3 amplicon, suggesting a potential role for additional gene products in 11q13-driven tumor progression [67]. Interestingly, mRNA for the cortactin binding partner SHANK2 was not significantly elevated, despite the presence of the SHANK2 gene within the amplicon and the validation of cortactin overexpression in the amplified cells [63,67].
A few studies have examined the function of cortactin in tumors grown in mice. In a spontaneous model of breast tumorigenesis, expression of cortactin under the MMTV promoter does not increase the number of new tumors, either in the absence or presence of cyclin D1 overexpression [68]. Thus, cortactin is unlikely to act as a tumor initiator. Since 11q13 amplification usually occurs as a late genetic change in cancer [9,69,70], this finding is not inconsistent with the known biology and suggests that cortactin expression may be most important in promoting tumor progression. Experimental metastasis experiments using tail vein injection of breast or esophageal squamous carcinoma cells demonstrated that overexpression of cortactin increased the number of metastases to bone [71] or lung [60], respectively. Overexpression of a cortactin with mutations in the src kinase phosphorylation sites led to decreased metastasis in the breast cancer model [71]. In a third study, orthotopic injection of hepatocellular carcinoma cells overexpressing cortactin into the liver led to increased intrahepatic metastasis, compared to control cells [72]. All three of these studies examined the role of cortactin in tumor growth. However, whereas an increase in tumor size was reported for subcutaneously-injected esophageal cancer cells [60], no change in size was found for tumors grown from orthotopically injected breast or hepatocellular cancer cells [71,72]. Thus, the strongest in vivo evidence is currently in support for a role for cortactin in metastasis, consistent with its well-characterized cell biological roles in cell motility and ECM degradation.
7. Posttranslational modification of cortactin
Cortactin is posttranslationally modified by many different kinases, including the tyrosine kinases src, fer, abl, fyn, syk, met, along with the serine/threonine kinases Erk, PAK, and MLCK [73,74]. Of these, the best characterized sites are in the C-terminus and include Y421, Y466, and Y482 that are phosphorylated by src family kinases [33] and S405 and S418 that are phosphorylated by Erk family kinases [27,75]. In the N-terminus, PAK phosphorylates cortactin on S113 in the first actin binding repeat, an interaction that leads to inhibition of actin-binding by cortactin [76]. Phosphorylation site mapping by mass spectrometry revealed additional sites, many of which are located in or near critical binding sites for the Arp2/3 complex and actin filaments [77].
Because cortactin was originally identified from a phosphotyrosine Western blot screen for src kinase substrates [1], the three src kinase phosphorylation sites [33] have been heavily studied. Those sites were subsequently shown to be phosphorylated by additional kinases, including Fer and Abl [74,78]. In vitro, the domain that contains these sites is not required for direct regulation of Arp2/3 activity by cortactin [14,15]. However, src phosphorylation may oppose the reported effect of Erk phosphorylation in promoting accessibility of the cortactin SH3 domain to binding partners such as N-WASp [27]. In addition, tyrosine phosphorylation of Y421 occurs first [79] and is thought to create an SH2 domain-binding site with affinity for additional signaling proteins such as Crk, Abl kinase, and Nck [80]. The interaction with Nck can lead to recruitment and activation of N-WASp by cortactin [28]. In cells, phosphorylation of cortactin by Abl is dependent on the presence of src kinases and is important for PDGF-induced dorsal wave formation [74]. However, since src kinase is important for separate activation of Abl downstream of growth factors [81,82], the requirement for src kinase may not be for direct phosphorylation of cortactin. Consistent with that concept, cortactin is more efficiently phosphorylated in vitro by Abl than by Src kinase [74].
Based on the importance of the cortactin C-terminus in binding to a variety of signaling and cytoskeletal proteins, the strength of C-terminal phosphorylation mutant phenotypes might be indicative of the relative importance of cortactin as a scaffolding molecule at various subcellular sites. For example, cortactin C-terminal domains and phosphorylation sites were found to be important for invasive functions such as podosome-associated ECM degradation and experimental metastasis [53,54,56,71], suggesting that ECM degradation may require cortactin to bring together C-terminal binding partners such as src kinase, dynamin, and N-WASp. By contrast, expression of C-terminal point or deletion mutants of cortactin have thus far given more modest and variable cell motility and adherens junction phenotypes than cortactin molecules with mutations in the N-terminal Arp2/3 complex or F-actin binding sites [16,31,33,35,41]. In these latter contexts, cortactin may function primarily to regulate branched actin stability or assembly through direct interactions with Arp2/3 complex and actin filaments.
Recently, posttranslational modification of the N-terminus has been reported. A number of phosphorylation sites are located in key regions, such as in the Arp2/3 binding acidic domain or in the critical 1st–4th actin binding repeats [18,76,77]. Most of the kinases responsible for these N-terminal phosphorylations are unknown, with the exception of PAK. The PAK phosphorylation site is located in the first actin-binding repeat and has been shown to modestly affect the affinity of cortactin for actin filaments [76] and regulate invadopodia activity in melanoma cells [56]. Interestingly, acetylation may also regulate actin assembly by cortactin, as HDAC6 was recently shown to affect the ability of cortactin to bind to actin filaments by modifying a charge patch in the repeats region [83].
8. Future prospects
In the 15 years since its identification as a src kinase substrate, cortactin has generated a great deal interest in the actin assembly and signaling fields. Indeed it appears to be a key regulator of branched actin assembly at a variety of subcellular sites, including in lamellipodia, invadopodia, cell-cell junctions, and at sites of vesicle trafficking. At this point, it is unclear which of the multiple potential mechanisms that have been demonstrated in vitro are operative in different cellular functions, e.g. direct or indirect activation of Arp2/3 complex, stabilization of branches and/or as a scaffolding protein. Careful studies with reexpression of mutant cortactin molecules in cortactin-deficient cells may provide some insight in this respect by demonstrating which binding partners are required for specific biochemical and cellular functions. Another important area of future research is likely to be investigation of the interactions between cortactin and cellular membranes, since cortactin may act as a bridge between branched actin and membrane trafficking molecules at a variety of sites.
In tumors, cortactin is frequently overexpressed, usually through 11q13 amplification. To date, there have been few studies examining the role of cortactin in tumor function. Of these, most support a strong role for cortactin in tumor metastasis [60,71,72] but not in tumor initiation [68]. However, more studies are needed to understand the role of cortactin in diverse tumor types and microenvironments. In addition, it is not clear which of the many cellular functions or binding domains of cortactin is operative in promoting in vivo aggressiveness. Finally, the role of cortactin in the context of 11q13-amplified tumors is not understood, since most studies have not used 11q13-amplified cells. Given the strong correlation of 11q13 amplification with poor patient prognosis, this is clearly an important area for future research. Ultimately, connecting the cell biology of cortactin function in a variety of contexts with in vivo tumor studies may lead to rational therapeutic strategies for patients with 11q13-amplified tumors.
Acknowledgements
This work was supported by NIH grants 1R01GM075126 and 1R21DE018244 to AMW. Thanks to Dr. Emily Clark for help with illustrations and for providing unpublished data, to Dr. Roberto Buccione for providing a preprint of his work, and to Dr. Tony Koleske for his input.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wu H, Reynolds AB, Kanner SB, Vines RR, Parsons JT. Identification and characterization of a novel cytoskeleton-associated pp60src substrate. Mol Cell Biol. 1991;11:5113–5124. doi: 10.1128/mcb.11.10.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu H, Parsons JT. Cortactin, an 80/85-kilodalton pp60src substrate, is a filamentous actin-binding protein enriched in the cell cortex. J Cell Biol. 1993;120:1417–1426. doi: 10.1083/jcb.120.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuuring E, Verhoeven E, Litvinov S, Michalides RJ. The product of the EMS1 gene, amplified and overexpressed in human carcinomas, is homologous to a v-src substrate and is located in cell-substratum contact sites. Mol Cell Biol. 1993;13:2891–2898. doi: 10.1128/mcb.13.5.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuuring E, Verhoeven E, Mooi WJ, Michalides RJ. Identification and cloning of two overexpressed genes, U21B31/PRAD1 and EMS1, within the amplified chromosome 11q13 region in human carcinomas. Oncogene. 1992;7:355–361. [PubMed] [Google Scholar]
- 5.Akervall JA, Jin Y, Wennerberg JP, Zatterstrom UK, Kjellen E, Mertens F, Willen R, Mandahl N, Heim S, Mitelman F. Chromosomal abnormalities involving 11q13 are associated with poor prognosis in patients with squamous cell carcinoma of the head and neck. Cancer. 1995;76:853–859. doi: 10.1002/1097-0142(19950901)76:5<853::aid-cncr2820760520>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 6.Akervall JA, Michalides RJ, Mineta H, Balm A, Borg A, Dictor MR, Jin Y, Loftus B, Mertens F, Wennerberg JP. Amplification of cyclin D1 in squamous cell carcinoma of the head and neck and the prognostic value of chromosomal abnormalities and cyclin D1 overexpression. Cancer. 1997;79:380–389. [PubMed] [Google Scholar]
- 7.Bockmuhl U, Schluns K, Kuchler I, Petersen S, Petersen I. Genetic imbalances with impact on survival in head and neck cancer patients. Am J Pathol. 2000;157:369–375. doi: 10.1016/S0002-9440(10)64549-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meredith SD, Levine PA, Burns JA, Gaffey MJ, Boyd JC, Weiss LM, Erickson NL, Williams ME. Chromosome 11q13 amplification in head and neck squamous cell carcinoma. Association with poor prognosis. Arch Otolaryngol Head Neck Surg. 1995;121:790–794. doi: 10.1001/archotol.1995.01890070076016. [DOI] [PubMed] [Google Scholar]
- 9.Myllykangas S, Bohling T, Knuutila S. Specificity, selection and significance of gene amplifications in cancer. Semin Cancer Biol. 2007;17:42–55. doi: 10.1016/j.semcancer.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Ormandy CJ, Musgrove EA, Hui R, Daly RJ, Sutherland RL. Cyclin D1, EMS1 and 11q13 amplification in breast cancer. Breast Cancer Res Treat. 2003;78:323–335. doi: 10.1023/a:1023033708204. [DOI] [PubMed] [Google Scholar]
- 11.Rodrigo JP, Garcia LA, Ramos S, Lazo PS, Suarez C. EMS1 gene amplification correlates with poor prognosis in squamous cell carcinomas of the head and neck. Clin Cancer Res. 2000;6:3177–3182. [PubMed] [Google Scholar]
- 12.Takes RP, Baatenburg de Jong RJ, Schuuring E, Hermans J, Vis AA, Litvinov SV, van Krieken JH. Markers for assessment of nodal metastasis in laryngeal carcinoma. Arch Otolaryngol Head Neck Surg. 1997;123:412–419. doi: 10.1001/archotol.1997.01900040048008. [DOI] [PubMed] [Google Scholar]
- 13.Williams ME, Gaffey MJ, Weiss LM, Wilczynski SP, Schuuring E, Levine PA. Chromosome 11Q13 amplification in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 1993;119:1238–1243. doi: 10.1001/archotol.1993.01880230084013. [DOI] [PubMed] [Google Scholar]
- 14.Uruno T, Liu J, Zhang P, Fan Yx Y, Egile C, Li R, Mueller SC, Zhan X. Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nat Cell Biol. 2001;3:259–266. doi: 10.1038/35060051. [DOI] [PubMed] [Google Scholar]
- 15.Weaver AM, Karginov AV, Kinley AW, Weed SA, Li Y, Parsons JT, Cooper JA. Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Curr Biol. 2001;11:370–374. doi: 10.1016/s0960-9822(01)00098-7. [DOI] [PubMed] [Google Scholar]
- 16.Bryce NS, Clark ES, Leysath JL, Currie JD, Webb DJ, Weaver AM. Cortactin promotes cell motility by enhancing lamellipodial persistence. Curr Biol. 2005;15:1276–1285. doi: 10.1016/j.cub.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 17.Katsube T, Togashi S, Hashimoto N, Ogiu T, Tsuji H. Filamentous actin binding ability of cortactin isoforms is responsible for their cell-cell junctional localization in epithelial cells. Arch Biochem Biophys. 2004;427:79–90. doi: 10.1016/j.abb.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 18.Weed SA, Karginov AV, Schafer DA, Weaver AM, Kinley AW, Cooper JA, Parsons JT. Cortactin localization to sites of actin assembly in lamellipodia requires interactions with F-actin and the Arp2/3 complex. J Cell Biol. 2000;151:29–40. doi: 10.1083/jcb.151.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goley ED, Welch MD. The ARP2/3 complex: an actin nucleator comes of age. Nat Rev Mol Cell Biol. 2006;7:713–726. doi: 10.1038/nrm2026. [DOI] [PubMed] [Google Scholar]
- 20.Higgs HN, Pollard TD. REGULATION OF ACTIN FILAMENT NETWORK FORMATION THROUGH ARP2/3 COMPLEX: Activation by a Diverse Array of Proteins. Annu Rev Biochem. 2001;70:649–676. doi: 10.1146/annurev.biochem.70.1.649. [DOI] [PubMed] [Google Scholar]
- 21.Higgs HN, Blanchoin L, Pollard TD. Influence of the C terminus of Wiskott-Aldrich syndrome protein (WASp) and the Arp2/3 complex on actin polymerization. Biochemistry. 1999;38:15212–15222. doi: 10.1021/bi991843+. [DOI] [PubMed] [Google Scholar]
- 22.Weaver AM, Heuser JE, Karginov AV, Lee WL, Parsons JT, Cooper JA. Interaction of cortactin and N-WASp with Arp2/3 complex. Curr Biol. 2002;12:1270–1278. doi: 10.1016/s0960-9822(02)01035-7. [DOI] [PubMed] [Google Scholar]
- 23.Egile C, Rouiller I, Xu XP, Volkmann N, Li R, Hanein D. Mechanism of filament nucleation and branch stability revealed by the structure of the Arp2/3 complex at actin branch junctions. PLoS Biol. 2005;3:e383. doi: 10.1371/journal.pbio.0030383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weed SA, Parsons JT. Cortactin: coupling membrane dynamics to cortical actin assembly. Oncogene. 2001;20:6418–6434. doi: 10.1038/sj.onc.1204783. [DOI] [PubMed] [Google Scholar]
- 25.Martin AC, Welch MD, Drubin DG. Arp2/3 ATP hydrolysis-catalysed branch dissociation is critical for endocytic force generation. Nat Cell Biol. 2006;8:826–833. doi: 10.1038/ncb1443. [DOI] [PubMed] [Google Scholar]
- 26.Kowalski JR, Egile C, Gil S, Snapper SB, Li R, Thomas SM. Cortactin regulates cell migration through activation of N-WASP. J Cell Sci. 2005;118:79–87. doi: 10.1242/jcs.01586. [DOI] [PubMed] [Google Scholar]
- 27.Martinez-Quiles N, Ho HY, Kirschner MW, Ramesh N, Geha RS. Erk/Src phosphorylation of cortactin acts as a switch on-switch off mechanism that controls its ability to activate N-WASP. Mol Cell Biol. 2004;24:5269–5280. doi: 10.1128/MCB.24.12.5269-5280.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tehrani S, Tomasevic N, Weed S, Sakowicz R, Cooper JA. Src phosphorylation of cortactin enhances actin assembly. Proc Natl Acad Sci U S A. 2007;104:11933–11938. doi: 10.1073/pnas.0701077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perrin BJ, Amann KJ, Huttenlocher A. Proteolysis of Cortactin by Calpain Regulates Membrane Protrusion during Cell Migration. Mol Biol Cell. 2006;17:239–250. doi: 10.1091/mbc.E05-06-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tehrani S, Faccio R, Chandrasekar I, Ross FP, Cooper JA. Cortactin has an essential and specific role in osteoclast actin assembly. Mol Biol Cell. 2006;17:2882–2895. doi: 10.1091/mbc.E06-03-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Helwani FM, Kovacs EM, Paterson AD, Verma S, Ali RG, Fanning AS, Weed SA, Yap AS. Cortactin is necessary for E-cadherin-mediated contact formation and actin reorganization. J Cell Biol. 2004;164:899–910. doi: 10.1083/jcb.200309034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel AS, Schechter GL, Wasilenko WJ, Somers KD. Overexpression of EMS1/cortactin in NIH3T3 fibroblasts causes increased cell motility and invasion in vitro. Oncogene. 1998;16:3227–3232. doi: 10.1038/sj.onc.1201850. [DOI] [PubMed] [Google Scholar]
- 33.Huang C, Liu J, Haudenschild CC, Zhan X. The role of tyrosine phosphorylation of cortactin in the locomotion of endothelial cells. J Biol Chem. 1998;273:25770–25776. doi: 10.1074/jbc.273.40.25770. [DOI] [PubMed] [Google Scholar]
- 34.Hill A, McFarlane S, Mulligan K, Gillespie H, Draffin JE, Trimble A, Ouhtit A, Johnston PG, Harkin DP, McCormick D, Waugh DJ. Cortactin underpins CD44- promoted invasion and adhesion of breast cancer cells to bone marrow endothelial cells. Oncogene. 2006;25:6079–6091. doi: 10.1038/sj.onc.1209628. [DOI] [PubMed] [Google Scholar]
- 35.Rothschild BL, Shim AH, Ammer AG, Kelley LC, Irby KB, Head JA, Chen L, Varella-Garcia M, Sacks PG, Frederick B, Raben D, Weed SA. Cortactin overexpression regulates actin-related protein 2/3 complex activity, motility, and invasion in carcinomas with chromosome 11q13 amplification. Cancer Res. 2006;66:8017–8025. doi: 10.1158/0008-5472.CAN-05-4490. [DOI] [PubMed] [Google Scholar]
- 36.van Rossum AG, Moolenaar WH, Schuuring E. Cortactin affects cell migration by regulating intercellular adhesion and cell spreading. Exp Cell Res. 2006;312:1658–1670. doi: 10.1016/j.yexcr.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 37.Somogyi K, Rorth P. Cortactin modulates cell migration and ring canal morphogenesis during Drosophila oogenesis. Mech Dev. 2004;121:57–64. doi: 10.1016/j.mod.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Kempiak SJ, Yamaguchi H, Sarmiento C, Sidani M, Ghosh M, Eddy RJ, Desmarais V, Way M, Condeelis J, Segall JE. A neural Wiskott-Aldrich Syndrome protein-mediated pathway for localized activation of actin polymerization that is regulated by cortactin. J Biol Chem. 2005;280:5836–5842. doi: 10.1074/jbc.M410713200. [DOI] [PubMed] [Google Scholar]
- 39.Unsworth KE, Way M, McNiven M, Machesky L, Holden DW. Analysis of the mechanisms of Salmonella-induced actin assembly during invasion of host cells and intracellular replication. Cell Microbiol. 2004;6:1041–1055. doi: 10.1111/j.1462-5822.2004.00417.x. [DOI] [PubMed] [Google Scholar]
- 40.El Sayegh TY, Arora PD, Fan L, Laschinger CA, Greer PA, McCulloch CA, Kapus A. Phosphorylation of N-cadherin-associated cortactin by Fer kinase regulates N-cadherin mobility and intercellular adhesion strength. Mol Biol Cell. 2005;16:5514–5527. doi: 10.1091/mbc.E05-05-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El Sayegh TY, Arora PD, Laschinger CA, Lee W, Morrison C, Overall CM, Kapus A, McCulloch CA. Cortactin associates with N-cadherin adhesions and mediates intercellular adhesion strengthening in fibroblasts. J Cell Sci. 2004;117:5117–5131. doi: 10.1242/jcs.01385. [DOI] [PubMed] [Google Scholar]
- 42.Cao H, Orth JD, Chen J, Weller SG, Heuser JE, McNiven MA. Cortactin is a component of clathrin-coated pits and participates in receptor-mediated endocytosis. Mol Cell Biol. 2003;23:2162–2170. doi: 10.1128/MCB.23.6.2162-2170.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao H, Weller S, Orth JD, Chen J, Huang B, Chen JL, Stamnes M, McNiven MA. Actin and Arf1-dependent recruitment of a cortactin-dynamin complex to the Golgi regulates post-Golgi transport. Nat Cell Biol. 2005;7:483–492. doi: 10.1038/ncb1246. [DOI] [PubMed] [Google Scholar]
- 44.Merrifield CJ, Perrais D, Zenisek D. Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells. Cell. 2005;121:593–606. doi: 10.1016/j.cell.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 45.Zhu J, Yu D, Zeng XC, Zhou K, Zhan X. Receptor-mediated endocytosis involves tyrosine phosphorylation of cortactin. J Biol Chem. 2007;282:16086–16094. doi: 10.1074/jbc.M701997200. [DOI] [PubMed] [Google Scholar]
- 46.Zhu J, Zhou K, Hao JJ, Liu J, Smith N, Zhan X. Regulation of cortactin/dynamin interaction by actin polymerization during the fission of clathrin-coated pits. J Cell Sci. 2005;118:807–817. doi: 10.1242/jcs.01668. [DOI] [PubMed] [Google Scholar]
- 47.Timpson P, Lynch DK, Schramek D, Walker F, Daly RJ. Cortactin overexpression inhibits ligand-induced down-regulation of the epidermal growth factor receptor. Cancer Res. 2005;65:3273–3280. doi: 10.1158/0008-5472.CAN-04-2118. [DOI] [PubMed] [Google Scholar]
- 48.Clark ES, Whigham AS, Yarbrough WG, Weaver AM. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res. 2007;67:4227–4235. doi: 10.1158/0008-5472.CAN-06-3928. [DOI] [PubMed] [Google Scholar]
- 49.Weaver AM. Invadopodia: specialized cell structures for cancer invasion. Clin Exp Metastasis. 2006;23:97–105. doi: 10.1007/s10585-006-9014-1. [DOI] [PubMed] [Google Scholar]
- 50.Linder S. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 2007;17:107–117. doi: 10.1016/j.tcb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 51.Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 2006;66:3034–3043. doi: 10.1158/0008-5472.CAN-05-2177. [DOI] [PubMed] [Google Scholar]
- 52.Bowden ET, Barth M, Thomas D, Glazer RI, Mueller SC. An invasion-related complex of cortactin, paxillin and PKCmu associates with invadopodia at sites of extracellular matrix degradation. Oncogene. 1999;18:4440–4449. doi: 10.1038/sj.onc.1202827. [DOI] [PubMed] [Google Scholar]
- 53.Webb BA, Eves R, Mak AS. Cortactin regulates podosome formation: roles of the protein interaction domains. Exp Cell Res. 2006;312:760–769. doi: 10.1016/j.yexcr.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 54.Webb BA, Jia L, Eves R, Mak AS. Dissecting the functional domain requirements of cortactin in invadopodia formation. Eur J Cell Biol. 2007;86:189–206. doi: 10.1016/j.ejcb.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 55.Yamaguchi H, Condeelis J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim Biophys Acta. 2007;1773:642–652. doi: 10.1016/j.bbamcr.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ayala I, Baldassarre M, Giacchetti G, Caldieri G, Tete S, Luini A, Buccione R. Multiple regulatory inputs converge on cortactin to control invadopodia biogenesis and extracellular matrix degradation. J Cell Sci. 2008;121:369–378. doi: 10.1242/jcs.008037. [DOI] [PubMed] [Google Scholar]
- 57.Bowden ET, Onikoyi E, Slack R, Myoui A, Yoneda T, Yamada KM, Mueller SC. Co-localization of cortactin and phosphotyrosine identifies active invadopodia in human breast cancer cells. Exp Cell Res. 2006 doi: 10.1016/j.yexcr.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 58.Clark ES, Weaver AM. "A new role for cortactin in invadopodia: regulation of protease secretion". Eur J Cell Biol. 2008 doi: 10.1016/j.ejcb.2008.01.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hui R, Ball JR, Macmillan RD, Kenny FS, Prall OW, Campbell DH, Cornish AL, McClelland RA, Daly RJ, Forbes JF, Blamey RW, Musgrove EA, Robertson JF, Nicholson RI, Sutherland RL. EMS1 gene expression in primary breast cancer: relationship to cyclin D1 and oestrogen receptor expression and patient survival. Oncogene. 1998;17:1053–1059. doi: 10.1038/sj.onc.1202023. [DOI] [PubMed] [Google Scholar]
- 60.Luo ML, Shen XM, Zhang Y, Wei F, Xu X, Cai Y, Zhang X, Sun YT, Zhan QM, Wu M, Wang MR. Amplification and overexpression of CTTN (EMS1) contribute to the metastasis of esophageal squamous cell carcinoma by promoting cell migration and anoikis resistance. Cancer Res. 2006;66:11690–11699. doi: 10.1158/0008-5472.CAN-06-1484. [DOI] [PubMed] [Google Scholar]
- 61.Yuan BZ, Zhou X, Zimonjic DB, Durkin ME, Popescu NC. Amplification and overexpression of the EMS 1 oncogene, a possible prognostic marker, in human hepatocellular carcinoma. J Mol Diagn. 2003;5:48–53. doi: 10.1016/S1525-1578(10)60451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Greer RO, Jr., Said S, Shroyer KR, Marileila VG, Weed SA. Overexpression of cyclin D1 and cortactin is primarily independent of gene amplification in salivary gland adenoid cystic carcinoma. Oral Oncol. 2007;43:735–741. doi: 10.1016/j.oraloncology.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 63.Freier K, Sticht C, Hofele C, Flechtenmacher C, Stange D, Puccio L, Toedt G, Radlwimmer B, Lichter P, Joos S. Recurrent coamplification of cytoskeleton-associated genes EMS1 and SHANK2 with CCND1 in oral squamous cell carcinoma. Genes Chromosomes Cancer. 2006;45:118–125. doi: 10.1002/gcc.20270. [DOI] [PubMed] [Google Scholar]
- 64.Schuuring E. The involvement of the chromosome 11q13 region in human malignancies: cyclin D1 and EMS1 are two new candidate oncogenes--a review. Gene. 1995;159:83–96. doi: 10.1016/0378-1119(94)00562-7. [DOI] [PubMed] [Google Scholar]
- 65.Hui R, Campbell DH, Lee CS, McCaul K, Horsfall DJ, Musgrove EA, Daly RJ, Seshadri R, Sutherland RL. EMS1 amplification can occur independently of CCND1 or INT-2 amplification at 11q13 and may identify different phenotypes in primary breast cancer. Oncogene. 1997;15:1617–1623. doi: 10.1038/sj.onc.1201311. [DOI] [PubMed] [Google Scholar]
- 66.Timpson P, Wilson AS, Lehrbach GM, Sutherland RL, Musgrove EA, Daly RJ. Aberrant expression of cortactin in head and neck squamous cell carcinoma cells is associated with enhanced cell proliferation and resistance to the epidermal growth factor receptor inhibitor gefitinib. Cancer Res. 2007;67:9304–9314. doi: 10.1158/0008-5472.CAN-07-0798. [DOI] [PubMed] [Google Scholar]
- 67.Gibcus JH, Menkema L, Mastik MF, Hermsen MA, de Bock GH, van Velthuysen ML, Takes RP, Kok K, Alvarez Marcos CA, van der Laan BF, van den Brekel MW, Langendijk JA, Kluin PM, van der Wal JE, Schuuring E. Amplicon mapping and expression profiling identify the Fas-associated death domain gene as a new driver in the 11q13.3 amplicon in laryngeal/pharyngeal cancer. Clin Cancer Res. 2007;13:6257–6266. doi: 10.1158/1078-0432.CCR-07-1247. [DOI] [PubMed] [Google Scholar]
- 68.van Rossum AG, van Bragt MP, Schuuring-Scholtes E, van der Ploeg JC, van Krieken JH, Kluin PM, Schuuring E. Transgenic mice with mammary gland targeted expression of human cortactin do not develop (pre-malignant) breast tumors: studies in MMTV-cortactin and MMTV-cortactin/-cyclin D1 bitransgenic mice. BMC Cancer. 2006;6:58. doi: 10.1186/1471-2407-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. N Engl J Med. 2001;345:1890–1900. doi: 10.1056/NEJMra001375. [DOI] [PubMed] [Google Scholar]
- 70.Hunter KD, Parkinson EK, Harrison PR. Profiling early head and neck cancer. Nat Rev Cancer. 2005;5:127–135. doi: 10.1038/nrc1549. [DOI] [PubMed] [Google Scholar]
- 71.Li Y, Tondravi M, Liu J, Smith E, Haudenschild CC, Kaczmarek M, Zhan X. Cortactin potentiates bone metastasis of breast cancer cells. Cancer Res. 2001;61:6906–6911. [PubMed] [Google Scholar]
- 72.Chuma M, Sakamoto M, Yasuda J, Fujii G, Nakanishi K, Tsuchiya A, Ohta T, Asaka M, Hirohashi S. Overexpression of cortactin is involved in motility and metastasis of hepatocellular carcinoma. J Hepatol. 2004;41:629–636. doi: 10.1016/j.jhep.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 73.Lua BL, Low BC. Cortactin phosphorylation as a switch for actin cytoskeletal network and cell dynamics control. FEBS Lett. 2005;579:577–585. doi: 10.1016/j.febslet.2004.12.055. [DOI] [PubMed] [Google Scholar]
- 74.Boyle SN, Michaud GA, Schweitzer B, Predki PF, Koleske AJ. A critical role for cortactin phosphorylation by Abl-family kinases in PDGF-induced dorsal-wave formation. Curr Biol. 2007;17:445–451. doi: 10.1016/j.cub.2007.01.057. [DOI] [PubMed] [Google Scholar]
- 75.Campbell DH, Sutherland RL, Daly RJ. Signaling pathways and structural domains required for phosphorylation of EMS1/cortactin. Cancer Res. 1999;59:5376–5385. [PubMed] [Google Scholar]
- 76.Webb BA, Zhou S, Eves R, Shen L, Jia L, Mak AS. Phosphorylation of cortactin by p21-activated kinase. Arch Biochem Biophys. 2006;456:183–193. doi: 10.1016/j.abb.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 77.Martin KH, Jeffery ED, Grigera PR, Shabanowitz J, Hunt DF, Parsons JT. Cortactin phosphorylation sites mapped by mass spectrometry. J Cell Sci. 2006;119:2851–2853. doi: 10.1242/jcs.03034. [DOI] [PubMed] [Google Scholar]
- 78.Sangrar W, Gao Y, Scott M, Truesdell P, Greer PA. Fer-mediated cortactin phosphorylation is associated with efficient fibroblast migration and is dependent on reactive oxygen species generation during integrin-mediated cell adhesion. Mol Cell Biol. 2007;27:6140–6152. doi: 10.1128/MCB.01744-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Head JA, Jiang D, Li M, Zorn LJ, Schaefer EM, Parsons JT, Weed SA. Cortactin tyrosine phosphorylation requires Rac1 activity and association with the cortical actin cytoskeleton. Mol Biol Cell. 2003;14:3216–3229. doi: 10.1091/mbc.E02-11-0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Okamura H, Resh MD. p80/85 cortactin associates with the Src SH2 domain and colocalizes with v-Src in transformed cells. J Biol Chem. 1995;270:26613–26618. doi: 10.1074/jbc.270.44.26613. [DOI] [PubMed] [Google Scholar]
- 81.Plattner R, Kadlec L, DeMali KA, Kazlauskas A, Pendergast AM. c-Abl is activated by growth factors and Src family kinases and has a role in the cellular response to PDGF. Genes Dev. 1999;13:2400–2411. doi: 10.1101/gad.13.18.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tanis KQ, Veach D, Duewel HS, Bornmann WG, Koleske AJ. Two distinct phosphorylation pathways have additive effects on Abl family kinase activation. Mol Cell Biol. 2003;23:3884–3896. doi: 10.1128/MCB.23.11.3884-3896.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang X, Yuan Z, Zhang Y, Yong S, Salas-Burgos A, Koomen J, Olashaw N, Parsons JT, Yang XJ, Dent SR, Yao TP, Lane WS, Seto E. HDAC6 modulates cell motility by altering the acetylation level of cortactin. Mol Cell. 2007;27:197–213. doi: 10.1016/j.molcel.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang R, Cao GJ, Guo H, Kordowska J, Albert CL. Wang, Direct interaction between caldesmon and cortactin. Arch Biochem Biophys. 2006;456:175–182. doi: 10.1016/j.abb.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hattan D, Nesti E, Cachero TG, Morielli AD. Tyrosine phosphorylation of Kv1.2 modulates its interaction with the actin-binding protein cortactin. J Biol Chem. 2002;277:38596–38606. doi: 10.1074/jbc.M205005200. [DOI] [PubMed] [Google Scholar]
- 86.Tian L, Chen L, McClafferty H, Sailer CA, Ruth P, Knaus HG, Shipston MJ. A noncanonical SH3 domain binding motif links BK channels to the actin cytoskeleton via the SH3 adapter cortactin. Faseb J. 2006;20:2588–2590. doi: 10.1096/fj.06-6152fje. [DOI] [PubMed] [Google Scholar]
- 87.Mizutani K, Miki H, He H, Maruta H, Takenawa T. Essential role of neural Wiskott-Aldrich syndrome protein in podosome formation and degradation of extracellular matrix in src-transformed fibroblasts. Cancer Res. 2002;62:669–674. [PubMed] [Google Scholar]
- 88.Kinley AW, Weed SA, Weaver AM, Karginov AV, Bissonette E, Cooper JA, Parsons JT. Cortactin Interacts with WIP in Regulating Arp2/3 Activation and Membrane Protrusion. Curr Biol. 2003;13:384–393. doi: 10.1016/s0960-9822(03)00107-6. [DOI] [PubMed] [Google Scholar]
- 89.Lin J, Liu J, Wang Y, Zhu J, Zhou K, Smith N, Zhan X. Differential regulation of cortactin and N-WASP-mediated actin polymerization by missing in metastasis (MIM) protein. Oncogene. 2005;24:2059–2066. doi: 10.1038/sj.onc.1208412. [DOI] [PubMed] [Google Scholar]
- 90.McNiven MA, Kim L, Krueger EW, Orth JD, Cao H, Wong TW. Regulated interactions between dynamin and the actin-binding protein cortactin modulate cell shape. J Cell Biol. 2000;151:187–198. doi: 10.1083/jcb.151.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Onodera Y, Hashimoto S, Hashimoto A, Morishige M, Mazaki Y, Yamada A, Ogawa E, Adachi M, Sakurai T, Manabe T, Wada H, Matsuura N, Sabe H. Expression of AMAP1, an ArfGAP, provides novel targets to inhibit breast cancer invasive activities. Embo J. 2005;24:963–973. doi: 10.1038/sj.emboj.7600588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Le Clainche C, Pauly BS, Zhang CX, Engqvist-Goldstein AE, Cunningham K, Drubin DG. A Hip1R-cortactin complex negatively regulates actin assembly associated with endocytosis. Embo J. 2007;26:1199–1210. doi: 10.1038/sj.emboj.7601576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Katsube T, Takahisa M, Ueda R, Hashimoto N, Kobayashi M, Togashi S. Cortactin associates with the cell-cell junction protein ZO-1 in both Drosophila and mouse. J Biol Chem. 1998;273:29672–29677. doi: 10.1074/jbc.273.45.29672. [DOI] [PubMed] [Google Scholar]
- 94.Dudek SM, Jacobson JR, Chiang ET, Birukov KG, Wang P, Zhan X, Garcia JG. Pulmonary endothelial cell barrier enhancement by sphingosine 1-phosphate: roles for cortactin and myosin light chain kinase. J Biol Chem. 2004;279:24692–24700. doi: 10.1074/jbc.M313969200. [DOI] [PubMed] [Google Scholar]
- 95.Du Y, Weed SA, Xiong WC, Marshall TD, Parsons JT. Identification of a novel cortactin SH3 domain-binding protein and its localization to growth cones of cultured neurons. Mol Cell Biol. 1998;18:5838–5851. doi: 10.1128/mcb.18.10.5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lynch DK, Winata SC, Lyons RJ, Hughes WE, Lehrbach GM, Wasinger V, Corthals G, Cordwell S, Daly RJ. A Cortactin-CD2-associated protein (CD2AP) complex provides a novel link between epidermal growth factor receptor endocytosis and the actin cytoskeleton. J Biol Chem. 2003;278:21805–21813. doi: 10.1074/jbc.M211407200. [DOI] [PubMed] [Google Scholar]
- 97.Lua BL, Low BC. BPGAP1 interacts with cortactin and facilitates its translocation to cell periphery for enhanced cell migration. Mol Biol Cell. 2004;15:2873–2883. doi: 10.1091/mbc.E04-02-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hou P, Estrada L, Kinley AW, Parsons JT, Vojtek AB, Gorski JL. Fgd1, the Cdc42 GEF responsible for Faciogenital Dysplasia, directly interacts with cortactin and mAbp1 to modulate cell shape. Hum Mol Genet. 2003;12:1981–1993. doi: 10.1093/hmg/ddg209. [DOI] [PubMed] [Google Scholar]
- 99.Ohoka Y, Takai Y. Isolation and characterization of cortactin isoforms and a novel cortactin-binding protein, CBP90. Genes Cells. 1998;3:603–612. doi: 10.1046/j.1365-2443.1998.00216.x. [DOI] [PubMed] [Google Scholar]