Abstract
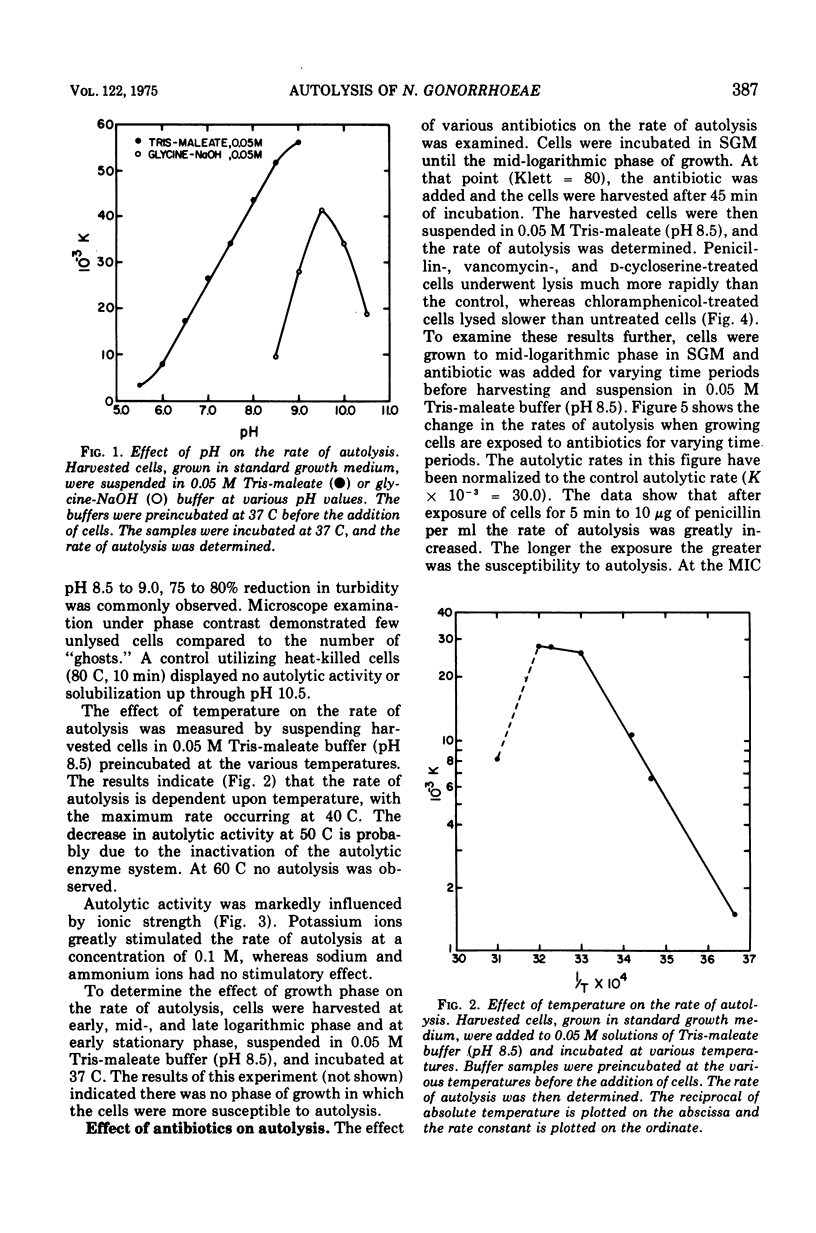
Physiological conditions that would provide maximal rates of autolysis of Neisseria gonorrhoeae were examined. Autolysis was found to occur over a broad pH range with the optimum at pH 9.0 IN 0.05 M tris(hydroxymethyl)amino-methane-maleate buffer. The temperature optimum was found to be 40 C. Potassium ions greatly stimulated autolysis at a concentration of 0.01 M. Exposure of growing N. gonorrhoeae cells to penicillin, vancomycin, or D-cycloserine influenced the susceptibility to the autolysis, whereas chloramphenicol afforded some protection against autolysis. The primary structure of the peptidoglycan is composed of muramic acid/glutamic acid/alanine/diaminopimelic acid/glucosamine in approximate molar ratios of 1:1:2:1:1, respectively. Exogenous radioactive diaminopimelic acid, D-glucosamine, and D-alanine were incorporated into peptidoglycan. During autolysis these radioactive fragments were released from cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brookes R., Hedén C. G. Dense cultures of Neisseria gonorrhoeae in liquid medium. Appl Microbiol. 1967 Mar;15(2):219–223. doi: 10.1128/am.15.2.219-223.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin B. W. Nutritional profiles of Neisseria gonorrhoeae, Neisseria meningitidis, and Neisseria lactamica in chemically defined media and the use of growth requirements for gonococcal typing. J Infect Dis. 1973 Aug;128(2):178–194. doi: 10.1093/infdis/128.2.178. [DOI] [PubMed] [Google Scholar]
- Ghuysen J. M. Use of bacteriolytic enzymes in determination of wall structure and their role in cell metabolism. Bacteriol Rev. 1968 Dec;32(4 Pt 2):425–464. [PMC free article] [PubMed] [Google Scholar]
- Gilpin R. W., Chatterjee A. N., Young F. E. Autolysis of microbial cells: salt activation of autolytic enzymes in a mutant of Staphylococcus aureus. J Bacteriol. 1972 Jul;111(1):272–283. doi: 10.1128/jb.111.1.272-283.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Procaryotic cell division with respect to wall and membranes. CRC Crit Rev Microbiol. 1971 May;1(1):29–72. doi: 10.3109/10408417109104477. [DOI] [PubMed] [Google Scholar]
- La Scolea L. J., Jr, Young F. E. Development of a defined minimal medium for the growth of Neisseria gonorrhoeae. Appl Microbiol. 1974 Jul;28(1):70–76. doi: 10.1128/am.28.1.70-76.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. P., Fleck J., Mock M., Ghuysen J. M. The wall peptidoglycans of Neisseria perflava, Moraxella glucidolytica, Pseudomonas alcaligenes and Proteus vulgaris strain P18. Eur J Biochem. 1973 Oct 5;38(2):301–306. doi: 10.1111/j.1432-1033.1973.tb03062.x. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Bartenstein L. Factors affecting autolysis of Neisseria gonorrhoeae. Proc Soc Exp Biol Med. 1974 Apr;145(4):1418–1421. doi: 10.3181/00379727-145-38025. [DOI] [PubMed] [Google Scholar]
- PELZER H. MUCOPEPTIDHYDROLASEN IN ESCHERICHIA COLI B. I. NACHWEIS UND WIRKUNGSSPEZIFITAET. Z Naturforsch B. 1963 Nov;18:950–956. [PubMed] [Google Scholar]
- Rogers H. J. Bacterial growth and the cell envelope. Bacteriol Rev. 1970 Jun;34(2):194–214. doi: 10.1128/br.34.2.194-214.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D. Symposium on the fine structure and replication of bacteria and their parts. IV. Unbalanced cell-wall synthesis: autolysis and cell-wall thickening. Bacteriol Rev. 1965 Sep;29(3):345–358. doi: 10.1128/br.29.3.345-358.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer J. D., Martin J. E., Jr Improved medium selective for cultivation of N. gonorrhoeae and N. meningitidis. Public Health Rep. 1966 Jun;81(6):559–562. [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Albino A., Zanati E. Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature. 1970 Jul 11;227(5254):138–140. doi: 10.1038/227138a0. [DOI] [PubMed] [Google Scholar]
- Tomasz A. Biological consequences of the replacement of choline by ethanolamine in the cell wall of Pneumococcus: chanin formation, loss of transformability, and loss of autolysis. Proc Natl Acad Sci U S A. 1968 Jan;59(1):86–93. doi: 10.1073/pnas.59.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]
- Young F. E. Autolytic enzyme associated with cell walls of Bacillus subtilis. J Biol Chem. 1966 Aug 10;241(15):3462–3467. [PubMed] [Google Scholar]



