Abstract
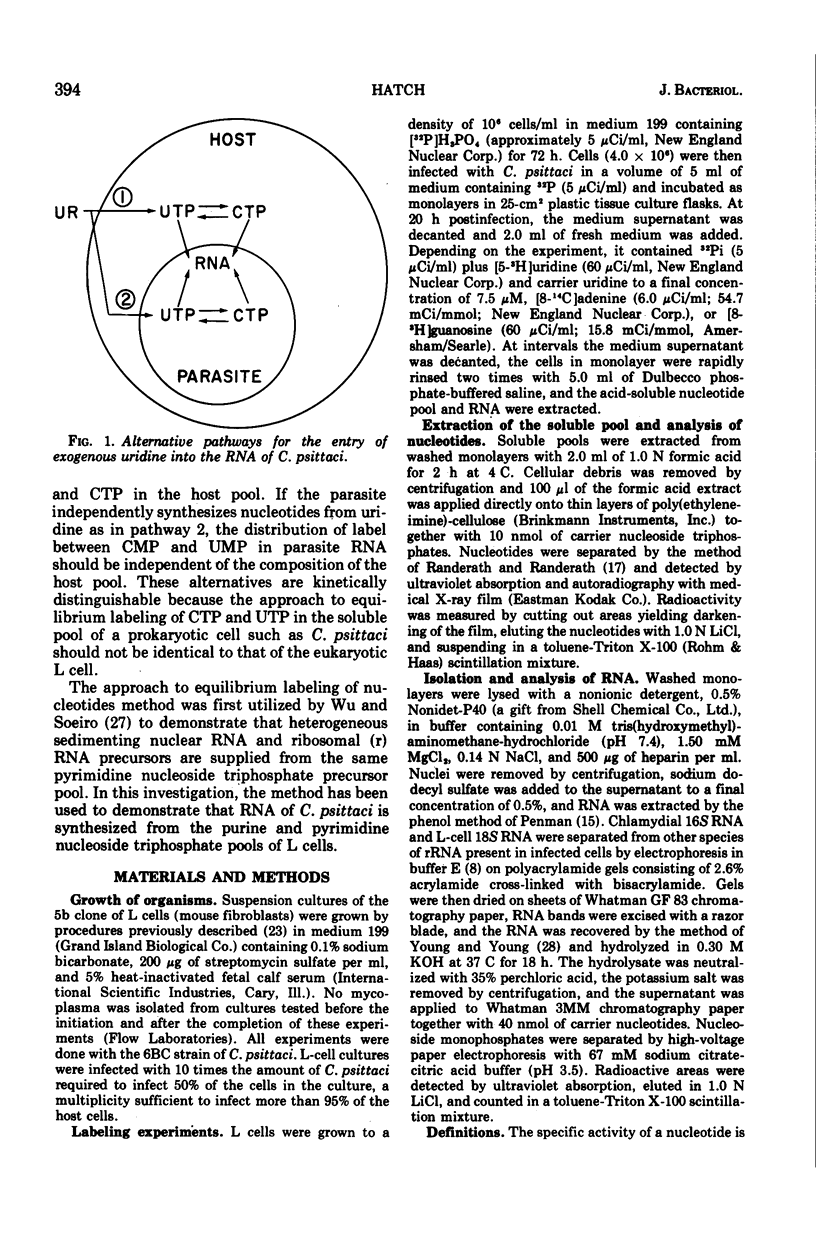
Long-term, 32-P-labeled L cells were infected with the obligately intracellular parasite Chlamydia psittaci (strain 6 BC). At 20 h postinfection, [3-H]uridine was added, and the infected cells were sampled at intervals for incorporation of the labels into the uridine triphosphate (UTP) and cytidine triphosphate (CTP) pools of the host L cell and the uridine monophosphate (UMP) and cytidine monophosphate (CMP) in 16S ribosomal ribonucleic acid (RNA) of the parasite. The specific activity of the nucleotides was calculated from the ratio of 3-H to 32-P counts in the nucleotides. The rate of approach to equilibrium labeling of UTP and CTP in L-cell pools and UMP and CMP in 16S RNA from the exogenous uridine label was determined from the increase in the ratios of the specific activities of CTP to UTP and CMP to UMP with time. The rate of approach to equilibrium CMP:UMP labeling of the 16S RNA of C. psittaci was consistent with the rate predicted from the kinetics of labeling of the CTP and UTP pools of the host L cell. In analogous experiments, the rate of approach to equilibrium guanosine monophosphate:adenosine monophosphate labeling of 16S RNA from an exogenous [14-C]adenine label was consistent with the rate predicted from the kinetics of labeling of the purine nucleoside triphosphate pool of the host cell. These results support the concept that members of the genus Chlamydia owe their obligate intracellular mode of reproduction to a requirement for energy intermediates which is fulfilled by the host cell. In addition, evidence was obtained that the total acid-soluble purine nucleoside triphosphate pool of L cells accurately represents the precursors of L-cell 18S ribosomal RNA.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. J. Effect of infection with the meningopneumonitis agent on deoxyribonucleic acid and protein synthesis by its L-cell host. J Bacteriol. 1969 Feb;97(2):653–657. doi: 10.1128/jb.97.2.653-657.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields T., Brox L. Specific activities of the UTP pools of human lymphocytes after uridine- 3 H labeling. Can J Biochem. 1973 Jun;51(6):954–957. doi: 10.1139/o73-121. [DOI] [PubMed] [Google Scholar]
- Gill S. D., Stewart R. B. Effect of metabolic inhibitors on the production of Chlamydia psittaci by infected L cells. Can J Microbiol. 1970 Nov;16(11):1079–1085. doi: 10.1139/m70-182. [DOI] [PubMed] [Google Scholar]
- Gill S. D., Stewart R. B. Respiration of L cells infected with Chlamydia psittaci. Can J Microbiol. 1970 Nov;16(11):1033–1039. doi: 10.1139/m70-175. [DOI] [PubMed] [Google Scholar]
- Kijima S., Wilt F. H. Rate of nuclear ribonucleic acid turnover in sea urchin embryos. J Mol Biol. 1969 Mar 14;40(2):235–246. doi: 10.1016/0022-2836(69)90472-0. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The determination of the molecular weight of ribonucleic acid by polyacrylamide-gel electrophresis. The effects of changes in conformation. Biochem J. 1969 Jun;113(1):131–138. doi: 10.1042/bj1130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGER J., MAGASANIK B. Guanosine 5'-phosphate reductase and its role in the interconversion of purine nucleotides. J Biol Chem. 1960 May;235:1474–1478. [PubMed] [Google Scholar]
- McCarthy B. J., Britten R. J. The Synthesis of Ribosomes in E. coli: I. The Incorporation of C-Uracil into the Metabolic Pool and RNA. Biophys J. 1962 Jan;2(1):35–47. doi: 10.1016/s0006-3495(62)86839-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W. The contribution of model systems to the understanding of infectious diseases. Perspect Biol Med. 1971 Spring;14(3):486–502. doi: 10.1353/pbm.1971.0024. [DOI] [PubMed] [Google Scholar]
- Nierlich D. P., Vielmetter W. Kinetic studies on the relationship of ribonucleotide precursor pools and ribonucleic acid synthesis. J Mol Biol. 1968 Feb 28;32(1):135–147. doi: 10.1016/0022-2836(68)90151-4. [DOI] [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G. Nucleotide pools of Novikoff rat hepatoma cells growing in suspension culture. II. Independent nucleotide pools for nucleic acid synthesis. J Cell Physiol. 1971 Apr;77(2):241–248. doi: 10.1002/jcp.1040770213. [DOI] [PubMed] [Google Scholar]
- RANDERATH E., RANDERATH K. RESOLUTION OF COMPLEX NUCLEOTIDE MIXTURES BY TWO-DIMENSIONAL ANION-EXCHANGE THIN-LAYER CHROMATOGRAPHY. J Chromatogr. 1964 Oct;16:126–129. doi: 10.1016/s0021-9673(01)82446-8. [DOI] [PubMed] [Google Scholar]
- Salser W., Janin J., Levinthal C. Measurement of the unstable RNA in exponentially growing cultures of Bacillus subtilis and Escherichia coli. J Mol Biol. 1968 Jan 28;31(2):237–266. doi: 10.1016/0022-2836(68)90442-7. [DOI] [PubMed] [Google Scholar]
- Sarov I., Becker Y. Deoxyribonucleic acid-dependent ribonucleic acid polymerase activity in purified trachoma elementary bodies: effect of sodium chloride on ribonucleic acid transcription. J Bacteriol. 1971 Sep;107(3):593–598. doi: 10.1128/jb.107.3.593-598.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeiro R., Ehrenfeld E. Cytoplasmic and nuclear pyrimidine ribonucleotide pools in HeLa cells. J Mol Biol. 1973 Jun 15;77(1):177–187. doi: 10.1016/0022-2836(73)90371-9. [DOI] [PubMed] [Google Scholar]
- Stokes G. V. Formation and destruction of internal membranes in L cells infected with Chlamydia psittaci. Infect Immun. 1973 Feb;7(2):173–177. doi: 10.1128/iai.7.2.173-177.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribby I. I. Cell Wall Synthesis by Chlamydia psittaci Growing in L Cells. J Bacteriol. 1970 Dec;104(3):1176–1188. doi: 10.1128/jb.104.3.1176-1188.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribby I. I., Moulder J. W. Availability of bases and nucleosides as precursors of nucleic acids in L cells and in the agent of meningopneumonitis. J Bacteriol. 1966 Jun;91(6):2362–2367. doi: 10.1128/jb.91.6.2362-2367.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Wilson N. N. Role of exogenous adenosine triphosphate in catabolic and synthetic activities of Chlamydia psittaci. J Bacteriol. 1969 Feb;97(2):719–724. doi: 10.1128/jb.97.2.719-724.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R. S., Soeiro R. Turnover of nuclear RNA in HeLa cells: evidence for a single ribonucleotide pool. J Mol Biol. 1971 Jun 14;58(2):481–487. doi: 10.1016/0022-2836(71)90365-2. [DOI] [PubMed] [Google Scholar]
- Young Y. P., Young R. J. A method for the recovery of nucleic acids from polyacrylamide gels. Anal Biochem. 1974 Mar;58(1):286–293. doi: 10.1016/0003-2697(74)90468-0. [DOI] [PubMed] [Google Scholar]


