Abstract
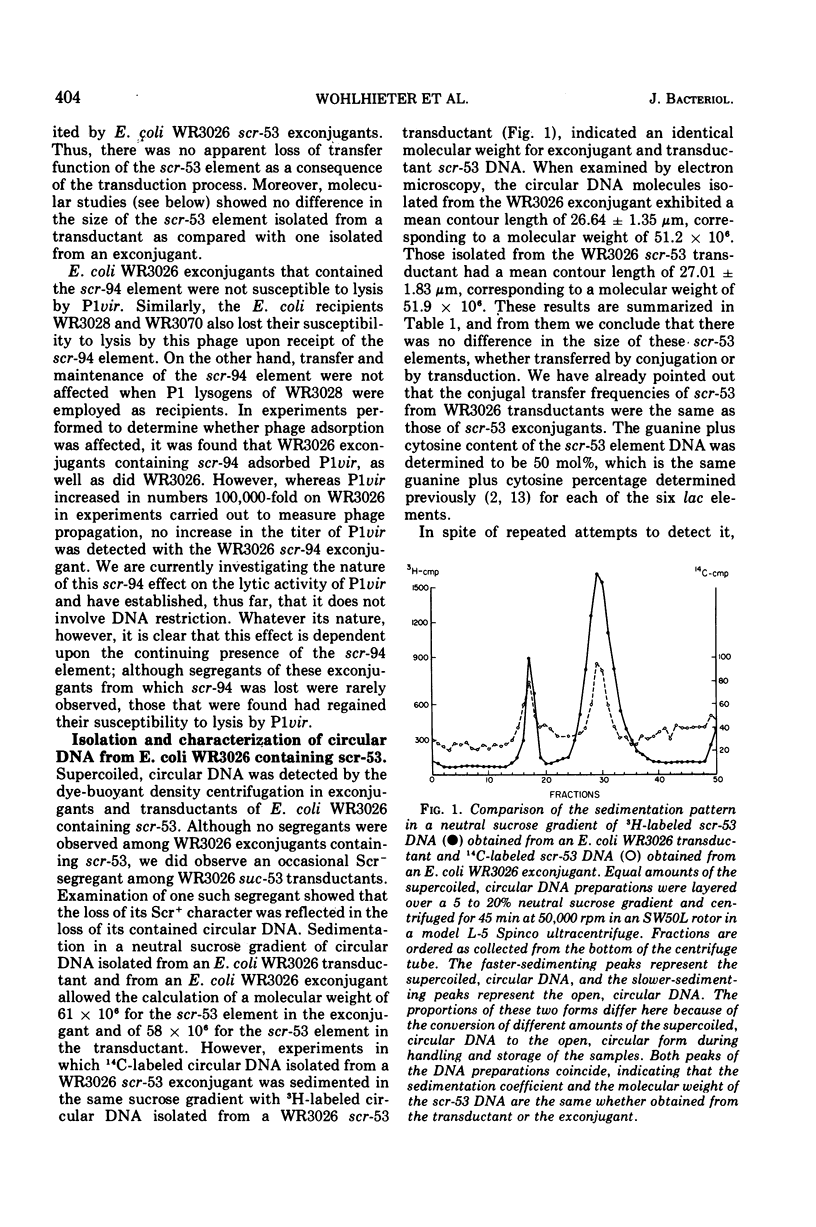
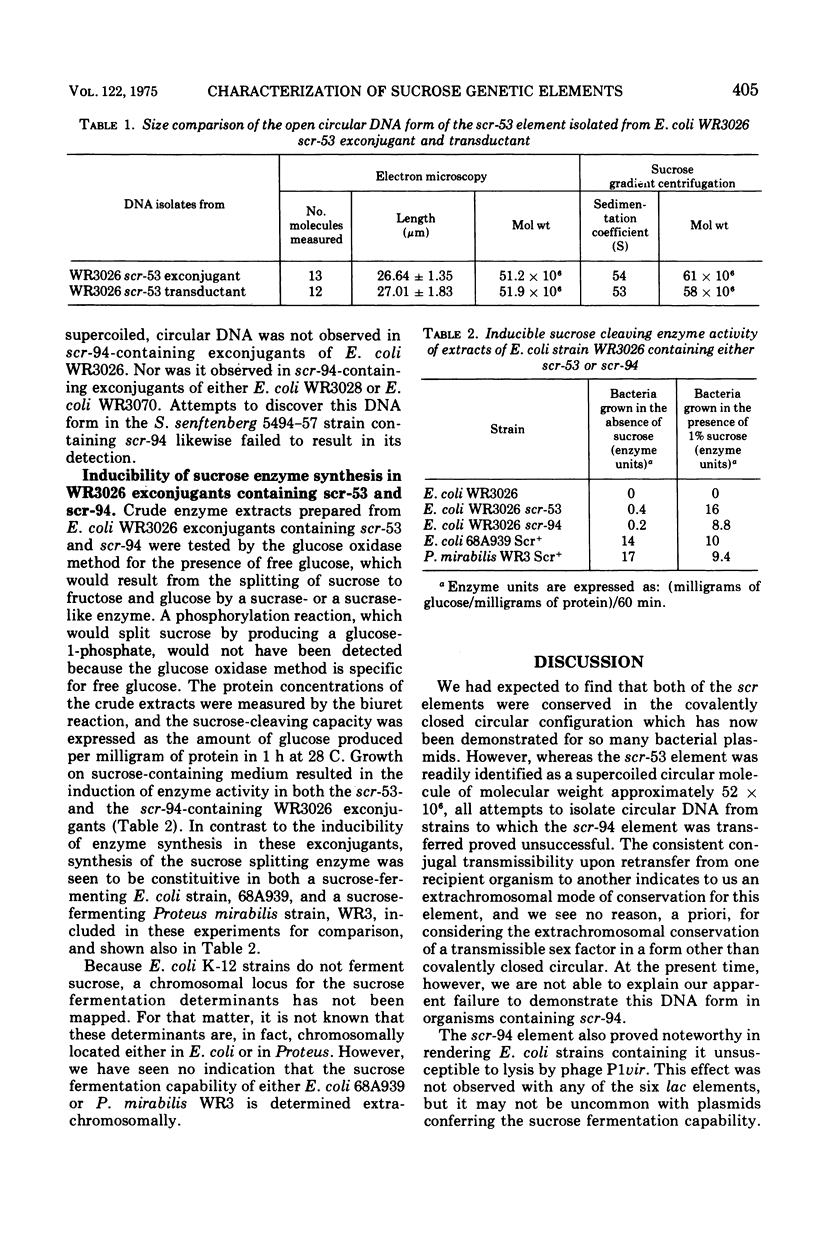
Two of seven sucrose-fermenting Salmonella strains obtained from clinical sources were found capable of conjugal transfer of the sucrose fermentation (Scr+) property to the Escherichia coli K-12 strain WR3026. The genetic elements conferring this Scr+ property, designated scr-53 and scr-94, were then conjugally transmissible from Escherichia coli WR3026 Scr+ exconjugants to other strains of Escherichia coli at frequences of 5 times 10- minus 6 to 5 times 10- minus 3 for the scr-53 element and 10- minus 6 to 10- minus 5 for the scr-94 element. In Escherichia coli hosts, both of these elements were compatible with F-lac and with each of six previously characterized transmissible lac elements. No antibiotic resistance characteristics or colicin production were discovered to be associated with either scr-53 or scr-94. Neither scr element generated a male host response to the female-specific phage phiII, but the scr-53 element rendered its Escherichia coli host sensitive to the male-specific phage R-17. Escherichia coli hosts containing scr-53 were susceptible to lysis by P1vir, and transduction of the scr-53 element was accomplished with this phage. The scr-53 element was isolated from Escherichia coli WR3026, Scr+ transductants, and Escherichia coli WR2036 Scr+ exconjugants as a covalently closed circular deoxyribonucleic acid molecule with a molecular weight (determined by electron microscopy) of approximately 52 times 10-6. Receipt of the scr-94 element rendered Escherichia coli hosts of this element unsusceptible to lysis by P1vir, although adsorption of the phage by an Escherichia coli WR3026 exconjugant containing scr-94 occurred as efficiently as it did on WR3026 itself. Repeated examination of Escherichia coli strains harboring scr-94, as well as of the Salmonella strain which initially contained it, did not reveal the presence of circular deoxyribonucleic acid. The synthesis of the sucrose cleaving enzyme was inducible in Escherichia coli exconjugants containing either scr-53 or scr-94.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Easterling S. B., Johnson E. M., Wohlhieter J. A., Baron L. S. Nature of lactose-fermenting Salmonella strains obtained from clinical sources. J Bacteriol. 1969 Oct;100(1):35–41. doi: 10.1128/jb.100.1.35-41.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FALKOW S., WOHLHIETER J. A., CITARELLA R. V., BARON L. S. TRANSFER OF EPISOMIC ELEMENTS TO PROTEUS. II. NATURE OF LAC+ PROTEUS STRAINS ISOLATED FROM CLINICAL SPECIMENS. J Bacteriol. 1964 Dec;88:1598–1601. doi: 10.1128/jb.88.6.1598-1601.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkow S., Baron L. S. EPISOMIC ELEMENT IN A STRAIN OF SALMONELLA TYPHOSA. J Bacteriol. 1962 Sep;84(3):581–589. doi: 10.1128/jb.84.3.581-589.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. M., Craig W. G., Jr, Wohlhieter J. A., Lazere J. R., Synenki R. M., Baron L. S. Conservation of Salmonella typhimurium deoxyribonucleic acid in partially diploid hybrids of Escherichia coli. J Bacteriol. 1973 Aug;115(2):629–634. doi: 10.1128/jb.115.2.629-634.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Minor L., Coynault C., Rohde R., Rowe B., Aleksic S. Localisation plasmidique du déterminant génétique du caractète atypique "saccharose plus" des Salmonella. Ann Microbiol (Paris) 1973 Oct;124(3):295–306. [PubMed] [Google Scholar]
- Leavitt R. W., Wohlhieter J. A., Johnson E. M., Olson G. E., Baron L. S. Isolation of circular deoxyribonucleic acid from Salmonella typhosa hybrids obtained from matings with Escherichia coli Hfr donors. J Bacteriol. 1971 Dec;108(3):1357–1365. doi: 10.1128/jb.108.3.1357-1365.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACHATTIE L. A., BERNE K. I., THOMAS C. A., Jr ELECTRON MICROSCOPY OF DNA FROM HEMOPHILUS INFLUENZAE. J Mol Biol. 1965 Mar;11:648–649. doi: 10.1016/s0022-2836(65)80019-5. [DOI] [PubMed] [Google Scholar]
- Neeley W. E., Wardlaw S. C., Sing H. C. Design and performance of a miniaturized high-speed continuous-flow analyzer. Clin Chem. 1974 Apr;20(4):424–427. [PubMed] [Google Scholar]
- Sharp P. A., Hsu M. T., Otsubo E., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. I. Structure of F-prime factors. J Mol Biol. 1972 Nov 14;71(2):471–497. doi: 10.1016/0022-2836(72)90363-4. [DOI] [PubMed] [Google Scholar]
- Synenki R. M., Wohlhieter J. A., Johnson E. M., Lazere J. R., Baron L. S. Isolation and characterization of circular deoxyribonucleic acid obtained from lactose-fermenting Salmonella strains. J Bacteriol. 1973 Dec;116(3):1185–1190. doi: 10.1128/jb.116.3.1185-1190.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOHLHIETER J. A., FALKOW S., CITARELLA R. V., BARON L. S. CHARACTERIZATION OF DNA FROM A PROTEUS STRAIN HARBORING AN EPISOME. J Mol Biol. 1964 Aug;9:576–588. doi: 10.1016/s0022-2836(64)80228-x. [DOI] [PubMed] [Google Scholar]


