Abstract
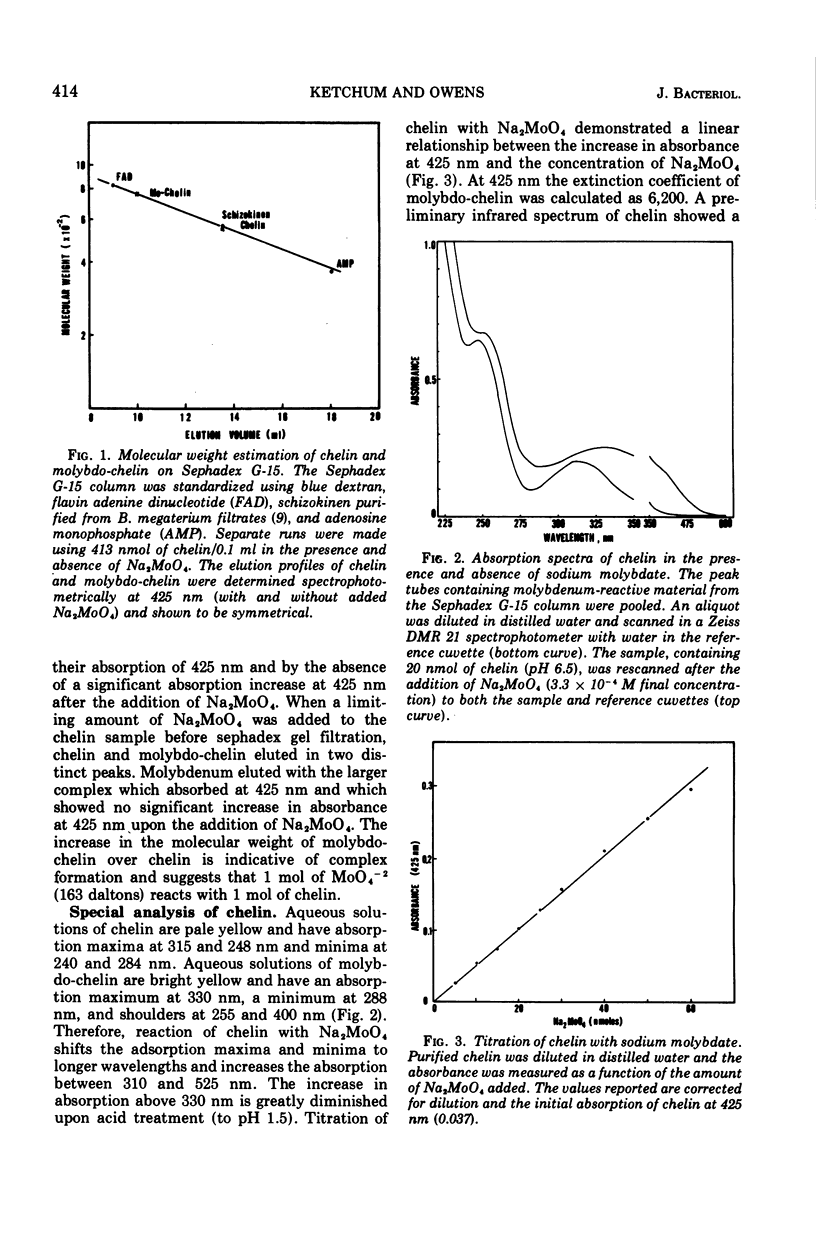
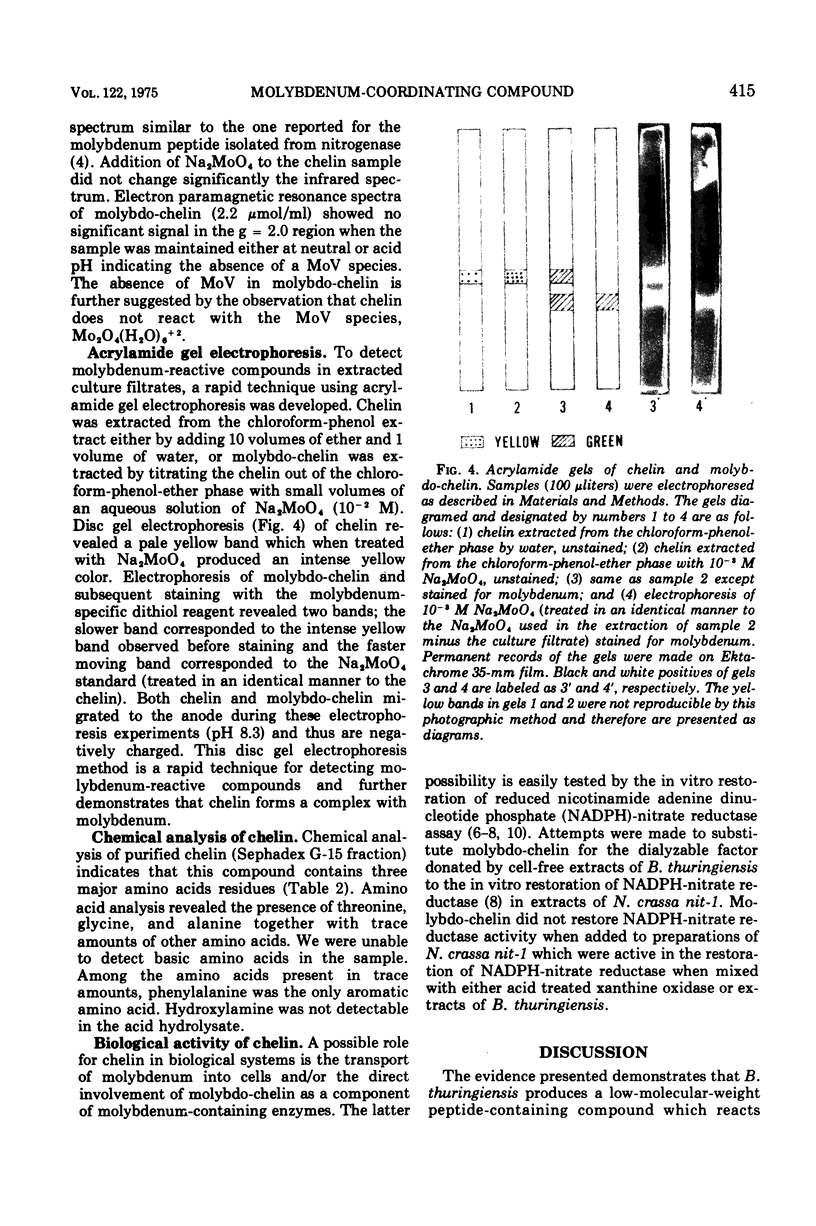
Bacillus thuringiensis (ATCC 10792) produces a molybdenum reactive compound (given the trivial name chelin) during growth on iron-deficient medium. This compound accumulates in the culture medium in direct relation to the amount of L-arginine added and reaches a maximum concentration 24 to 48 h after the stationary phase of growth. Chelin absorbs light in the ultraviolet region with absorption maxima at 315 and 248 nm and minima at 284 and 240 nm. Chelin reacts with Na2MoO4, but not with Mo2O4(H2O)6-2+, to form a bright yellow molybdo-chelin complex which absorbs light with an absorption maximum at 330 nm, a minimum at 288 nm, and shoulders at 255 and 400 nm. The differential absorption of molybdo-chelin versus chelin at 425 nm can be used to quantify chelin. This differential absorbance is linear with increasing concentrations of Na2MoO4 and was used to calculate the molar extinction coefficient of molybdochelin at 425 nm (epsilon similar to 6,200). Chelin binds MoO4-2 minus to form a complex (molybdochelin) which migrates as a single band and elutes as a single peak, during acrylamide gel electrophoresis and Sephadex G-15 gel filtration. Molecular weight determinations using Sephadex G-15 gel filtration resulted in an estimated molecular weight of 550 for chelin and an estimated molecular weight of 760 for molybdo-chelin. The peptide nature of chelin is indicated by its positive ninhydrin reaction on thin-layer chromatography plates and by the presence of amino acids in acid-hydrolyzed samples. The major amino acid residues detected were threonine, glycine, and alanine.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Ganelin V. L., L'vov N. P., Sergeev N. S., Shaposhnikov G. L., Kretovich V. L. Vydelenie i svoistva molibdensoderzhashchego peptida iz komponenta I azotfiksiruiushchego kompleksa Azotobacter vinelandii. Dokl Akad Nauk SSSR. 1972 Oct 11;206(5):1236–1238. [PubMed] [Google Scholar]
- Ketchum P. A., Cambier H. Y., Frazier W. A., 3rd, Madansky C. H., Nason A. In vitro assembly of Neurospora assimilatory nitrate reductase from protein subunits of a Neurospora mutant and the xanthine oxidizing or aldehyde oxidase systems of higher animals. Proc Natl Acad Sci U S A. 1970 Jul;66(3):1016–1023. doi: 10.1073/pnas.66.3.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketchum P. A., Sevilla C. L. In vitro formation of nitrate reductase using extracts of the nitrate reductase mutant of Neurospora crassa, nit-1, and Rhodospirillum rubrum. J Bacteriol. 1973 Nov;116(2):600–609. doi: 10.1128/jb.116.2.600-609.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketchum P. A., Swarin R. S. In vitro formation of assimilatory nitrate reductase: presence of the constitutive component in bacteria. Biochem Biophys Res Commun. 1973 Jun 19;52(4):1450–1456. doi: 10.1016/0006-291x(73)90663-3. [DOI] [PubMed] [Google Scholar]
- Mullis K. B., Pollack J. R., Neilands J. B. Structure of schizokinen, an iron-transport compound from Bacillus megaterium. Biochemistry. 1971 Dec 21;10(26):4894–4898. doi: 10.1021/bi00802a010. [DOI] [PubMed] [Google Scholar]
- Nason A., Antoine A. D., Ketchum P. A., Frazier W. A., 3rd, Lee D. K. Formation of assimilatory nitrate reductase by in vitro inter-cistronic complementation in Neurospora crassa. Proc Natl Acad Sci U S A. 1970 Jan;65(1):137–144. doi: 10.1073/pnas.65.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATEMAN J. A., COVE D. J., REVER B. M., ROBERTS D. B. A COMMON CO-FACTOR FOR NITRATE REDUCTASE AND XANTHINE DEHYDROGENASE WHICH ALSO REGULATES THE SYNTHESIS OF NITRATE REDUCTASE. Nature. 1964 Jan 4;201:58–60. doi: 10.1038/201058a0. [DOI] [PubMed] [Google Scholar]
- Werner D., Russell S. A., Evans H. J. Reduction of acetylene and hydrazine with a molybdenum-glutathione complex. Proc Natl Acad Sci U S A. 1973 Feb;70(2):339–342. doi: 10.1073/pnas.70.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]




