Abstract
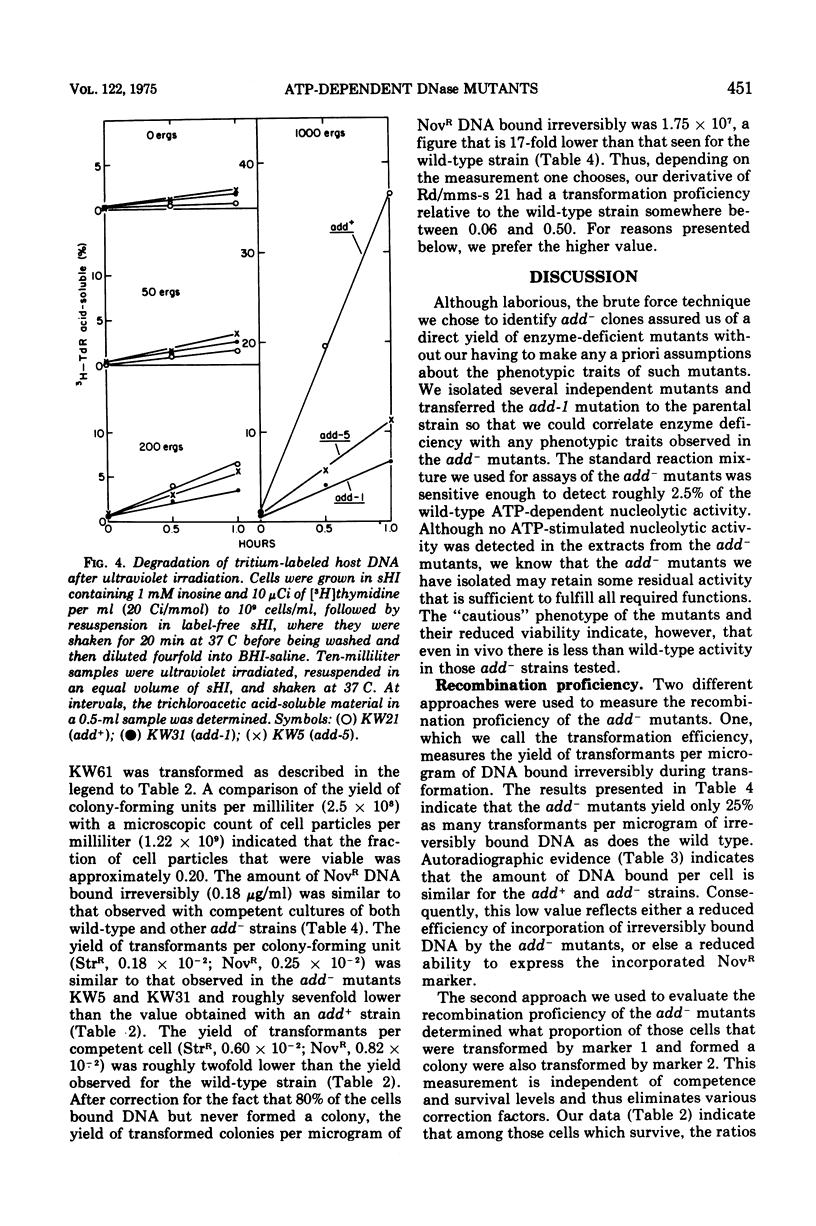
By a direct assay approach, mutants of Haemophilus influenzae Rd that are deficient in adenosine 5'-triphosphate-dependent deoxyribonuclease activity (add-) were isolated and characterized. A large proportion (50 to 90%) of the cells in cultures of these mutants failed to produce visible colonies when plated. An extensive analysis of the recombination proficiency of these strains revealed that the transformation frequency (transformants per competent cell) in the mutants was similar to that found in the wild type, but that the transformation efficiency (transformants per microgram of irreversibly bound deoxyribonucleic acid [DNA]) was reduced approximately fourfold. Sensitivities of the mutants to gamma rays, ultraviolet radiation, and methyl methane sulfonate were only slightly greater than wild-type levels. The rate of degradation of host DNA after ultraviolet irradiation was significantly reduced in the mutants. It is suggested that the adenosine 5'-triphosphate-dependent deoxyribonuclease in H. influenzae plays a nonessential role in DNA recombination and repair.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buttin G., Wright M. Enzymatic DNA degradation in E. coli: its relationship to synthetic processes at the chromosome level. Cold Spring Harb Symp Quant Biol. 1968;33:259–269. doi: 10.1101/sqb.1968.033.01.030. [DOI] [PubMed] [Google Scholar]
- Caster J. H., Goodgal S. H. Competence Mutant of Haemophilus influenzae with Abnormal Ratios of Marker Efficiencies in Transformation. J Bacteriol. 1972 Oct;112(1):492–502. doi: 10.1128/jb.112.1.492-502.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caster J. H., Postel E. H., Goodgal S. H. Competence mutants: isolation of transformation deficient strains of Haemophilus influenzae. Nature. 1970 Aug 1;227(5257):515–517. doi: 10.1038/227515a0. [DOI] [PubMed] [Google Scholar]
- Chestukhin A. V., Shemyakin M. F., Kalinina N. A., Prozorov A. A. Some properties of ATP dependent deoxyribonucleases from normal and rec-mutant strains of Bacillus subtilis. FEBS Lett. 1972 Jul 15;24(1):121–125. doi: 10.1016/0014-5793(72)80841-x. [DOI] [PubMed] [Google Scholar]
- Clark A. J. Toward a metabolic interpretation of genetic recombination of E. coli and its phages. Annu Rev Microbiol. 1971;25:437–464. doi: 10.1146/annurev.mi.25.100171.002253. [DOI] [PubMed] [Google Scholar]
- Doly J., Sasarman E., Anagnostopoulos C. ATP-dependent deoxyribonuclease in Bacillus subtilis and a mutant deficient in this activity. Mutat Res. 1974 Jan;22(1):15–23. doi: 10.1016/0027-5107(74)90003-7. [DOI] [PubMed] [Google Scholar]
- Friedman E. A., Smith H. O. An adenosine triphosphate-dependent deoxyribonuclease from Hemophilus influenzae Rd. 3. Substrate specificity. J Biol Chem. 1972 May 10;247(9):2859–2865. [PubMed] [Google Scholar]
- Friedman E. A., Smith H. O. An adenosine triphosphate-dependent deoxyribonuclease from Hemophilus influenzae Rd. I. Purification and properties of the enzyme. J Biol Chem. 1972 May 10;247(9):2846–2853. [PubMed] [Google Scholar]
- Friedman E. A., Smith H. O. Production of possible recombination intermediates by an ATP-dependent DNAase. Nat New Biol. 1973 Jan 10;241(106):54–58. doi: 10.1038/newbio241054a0. [DOI] [PubMed] [Google Scholar]
- GOODGAL S. H., HERRIOTT R. M. Studies on transformations of Hemophilus influenzae. I. Competence. J Gen Physiol. 1961 Jul;44:1201–1227. doi: 10.1085/jgp.44.6.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greth M. L., Chevallier M. R. Studies on ATP-dependent deoxyribonuclease of Haemophilus influenzae: involvement of the enzyme in the transformation process. Biochem Biophys Res Commun. 1973 Sep 5;54(1):1–8. doi: 10.1016/0006-291x(73)90880-2. [DOI] [PubMed] [Google Scholar]
- Haefner K. Spontaneous lethal sectoring, a further feature of Escherichia coli strains deficient in the function of rec and uvr genes. J Bacteriol. 1968 Sep;96(3):652–659. doi: 10.1128/jb.96.3.652-659.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herriott R. M., Meyer E. Y., Vogt M., Modan M. Defined medium for growth of Haemophilus influenzae. J Bacteriol. 1970 Feb;101(2):513–516. doi: 10.1128/jb.101.2.513-516.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P. DNA repair and genetic recombination: studies on mutants of Escherichia coli defective in these processes. Radiat Res. 1966;(Suppl):156+–156+. [PubMed] [Google Scholar]
- Howard-Flanders P. DNA repair. Annu Rev Biochem. 1968;37:175–200. doi: 10.1146/annurev.bi.37.070168.001135. [DOI] [PubMed] [Google Scholar]
- Kooistra J., Venema G. Fate of donor DNA in some poorly transformable strains of Haemophilus influenzae. Mutat Res. 1970 Mar;9(3):245–253. doi: 10.1016/0027-5107(70)90126-0. [DOI] [PubMed] [Google Scholar]
- Lark K. G., Lark C. Regulation of chromosome replication in Escherichia coli: alternate replication of two chromosomes at slow growth rates. J Mol Biol. 1965 Aug;13(1):105–126. doi: 10.1016/s0022-2836(65)80083-3. [DOI] [PubMed] [Google Scholar]
- Milcarek C., Weiss B. Mutants of Escherichia coli with altered deoxyribonucleases. I. Isolation and characterization of mutants for exonuclease 3. J Mol Biol. 1972 Jul 21;68(2):303–318. doi: 10.1016/0022-2836(72)90215-x. [DOI] [PubMed] [Google Scholar]
- Oishi M. An ATP-dependent deoxyribonuclease from Escherichia coli with a possible role in genetic recombination. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1292–1299. doi: 10.1073/pnas.64.4.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. D., Guild W. R. Number of transformable units per cell in Diplococcus pneumoniae. J Bacteriol. 1969 Mar;97(3):1033–1035. doi: 10.1128/jb.97.3.1033-1035.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scocca J. J., Poland R. L., Zoon K. C. Specificity in deoxyribonucleic acid uptake by transformable Haemophilus influenzae. J Bacteriol. 1974 May;118(2):369–373. doi: 10.1128/jb.118.2.369-373.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Brown D. C., Boling M. E., Mattingly A., Gordon M. P. Repair of deoxyribonucleic acid in Haemophilus influenzae. I. X-ray sensitivity of ultraviolet-sensitive mutants and their behavior as hosts to ultraviolet-irradiated bacteriophage and transforming deoxyribonucleic acid. J Bacteriol. 1968 Feb;95(2):546–558. doi: 10.1128/jb.95.2.546-558.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Friedman E. A. An adenosine triphosphate-dependent deoxyribonuclease from Hemophilus influenzae Rd. II. Adenosine triphosphatase properties. J Biol Chem. 1972 May 10;247(9):2854–2858. [PubMed] [Google Scholar]
- Smith H. O., Wilcox K. W. A restriction enzyme from Hemophilus influenzae. I. Purification and general properties. J Mol Biol. 1970 Jul 28;51(2):379–391. doi: 10.1016/0022-2836(70)90149-x. [DOI] [PubMed] [Google Scholar]
- TSUDA Y., STRAUSS B. S. A DEOXYRIBONUCLEASE REACTION REQUIRING NUCLEOSIDE DI- OR TRIPHOSPHATES. Biochemistry. 1964 Nov;3:1678–1684. doi: 10.1021/bi00899a013. [DOI] [PubMed] [Google Scholar]
- Vovis G. F. Adenosine triphosphate-dependent deoxyribonuclease from Diplococcus pneumoniae: fate of transforming deoxyribonucleic acid in a strain deficient in the enzymatic activity. J Bacteriol. 1973 Feb;113(2):718–723. doi: 10.1128/jb.113.2.718-723.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vovis G. F., Buttin G. An ATP-dependent deoxyribonuclease from Diplococcus pneumoniae. II. Evidence for its involvement in bacterial recombination. Biochim Biophys Acta. 1970 Nov 12;224(1):42–54. [PubMed] [Google Scholar]
- Willetts N. S., Clark A. J., Low B. Genetic location of certain mutations conferring recombination deficiency in Escherichia coli. J Bacteriol. 1969 Jan;97(1):244–249. doi: 10.1128/jb.97.1.244-249.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright M., Buttin G. Les mécanismes de dégradation enzymatique du chromosome bactérien et leur régulation. Bull Soc Chim Biol (Paris) 1969;51(10):1373–1383. [PubMed] [Google Scholar]


