Abstract
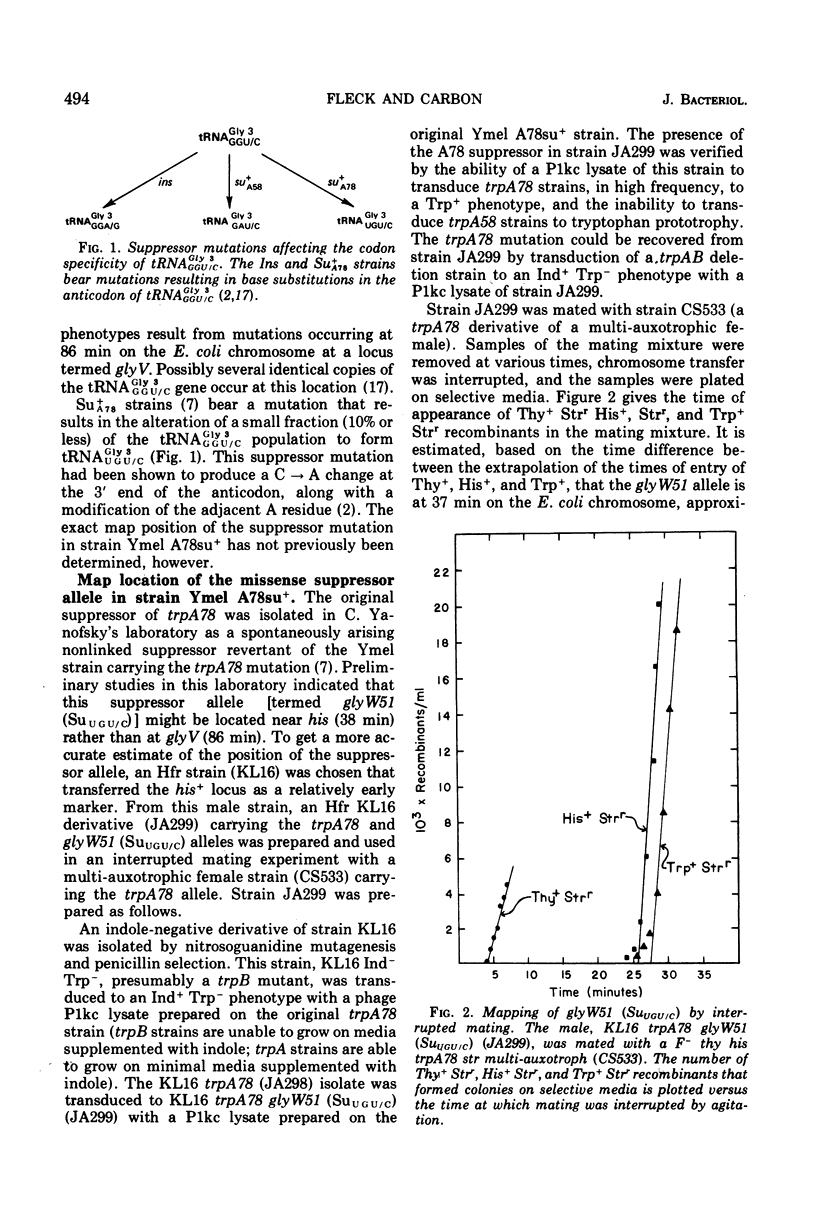
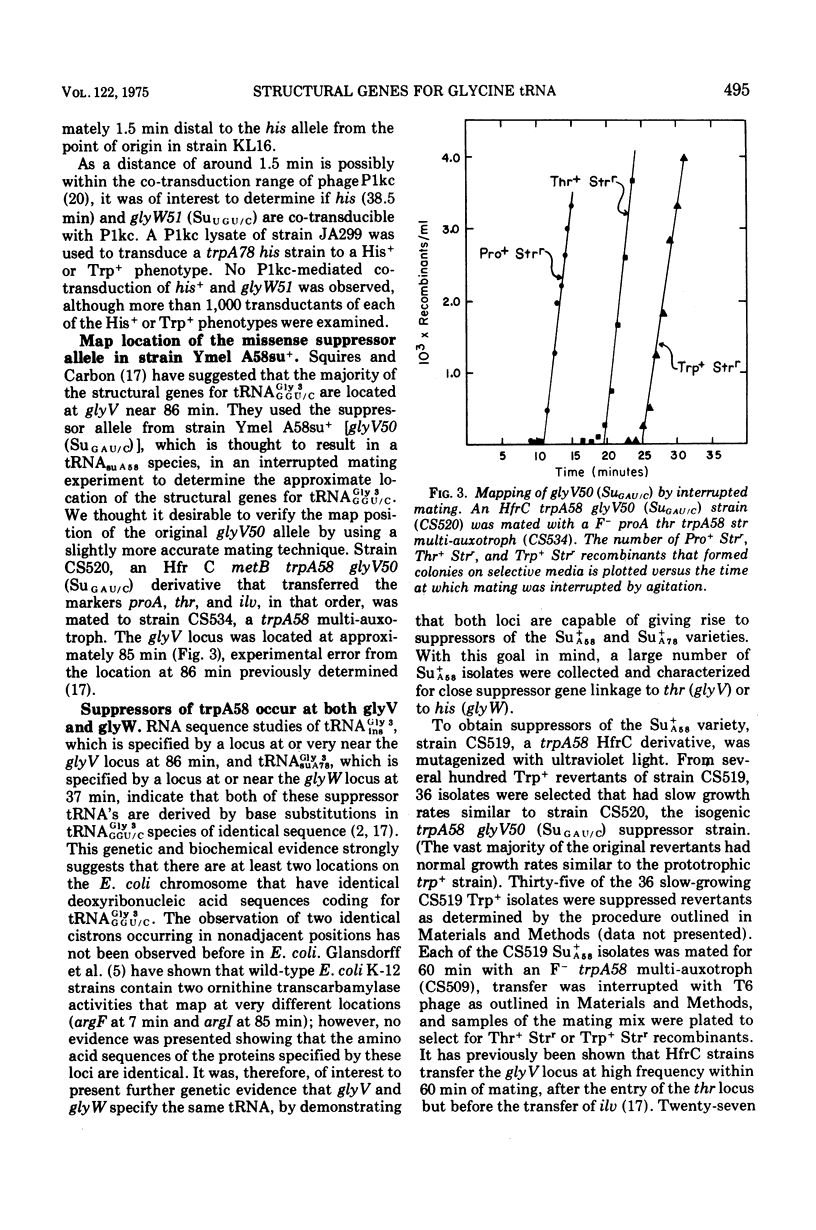
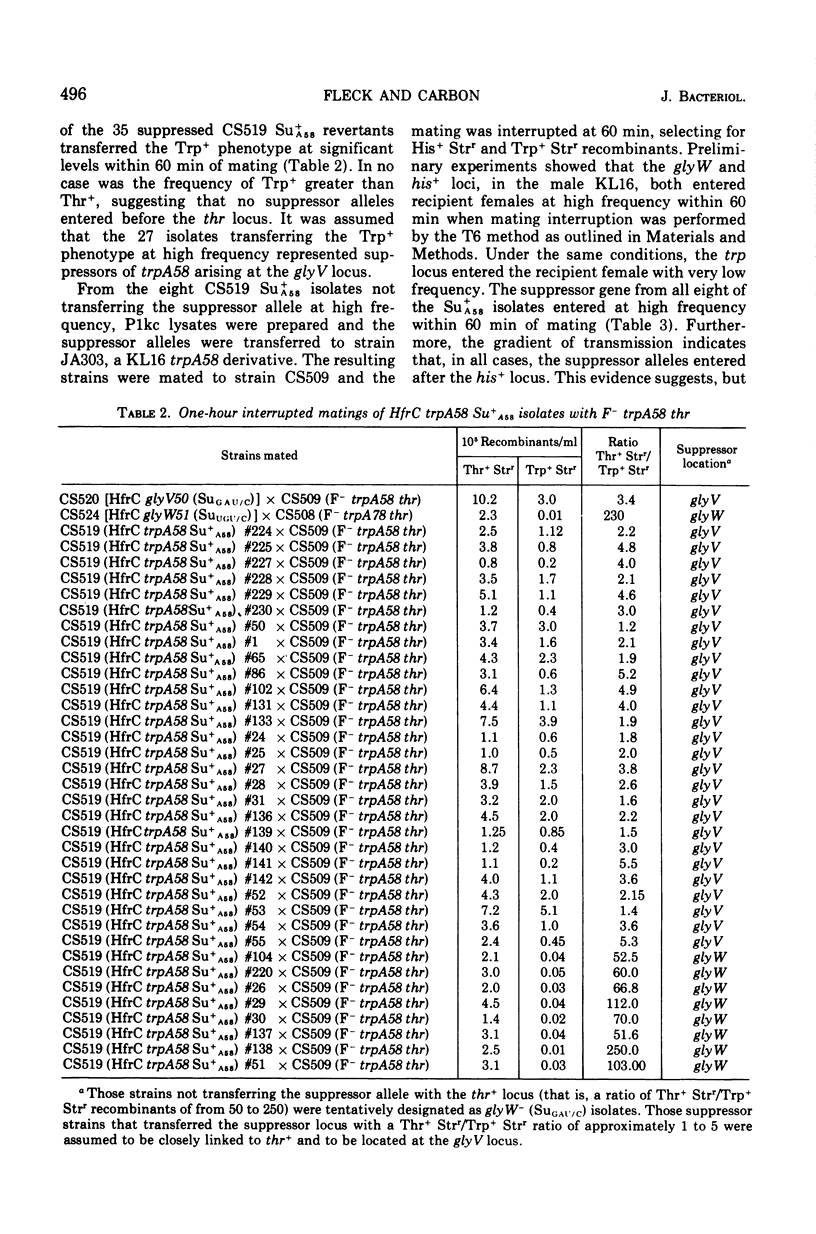
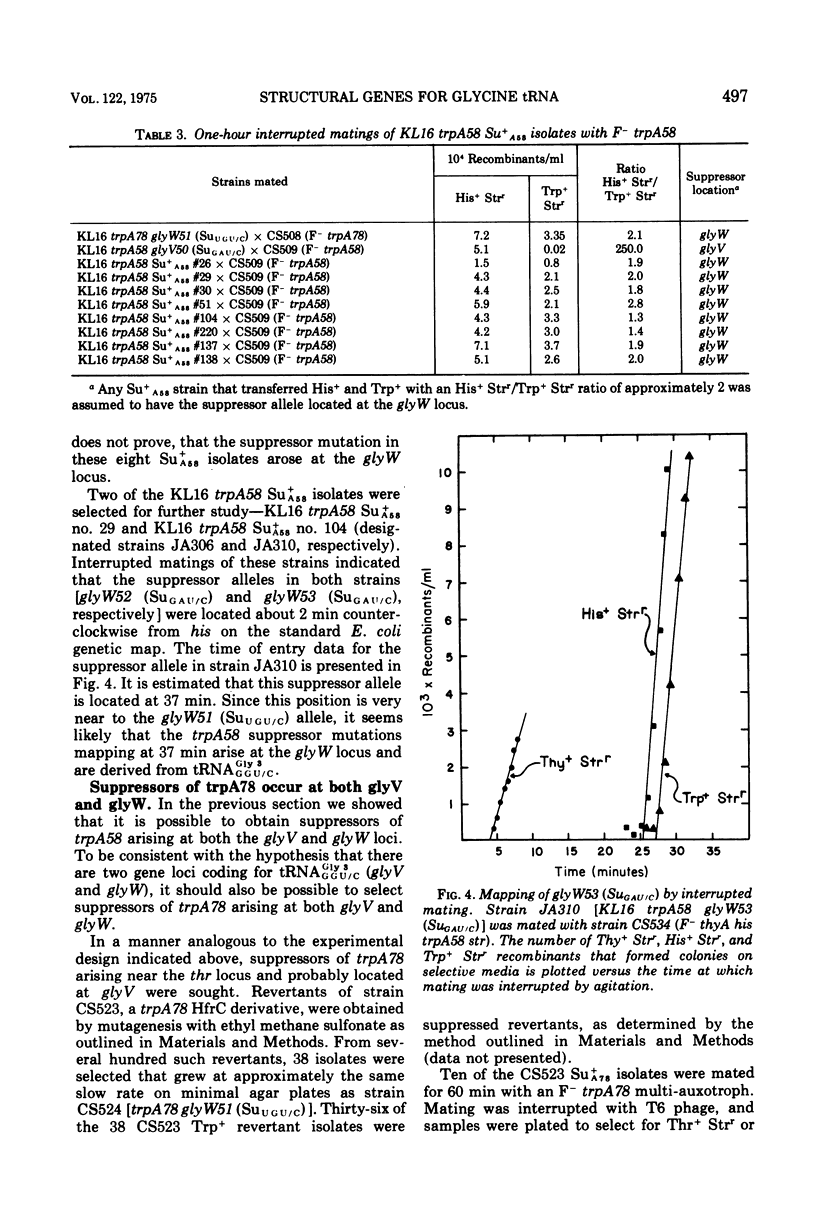
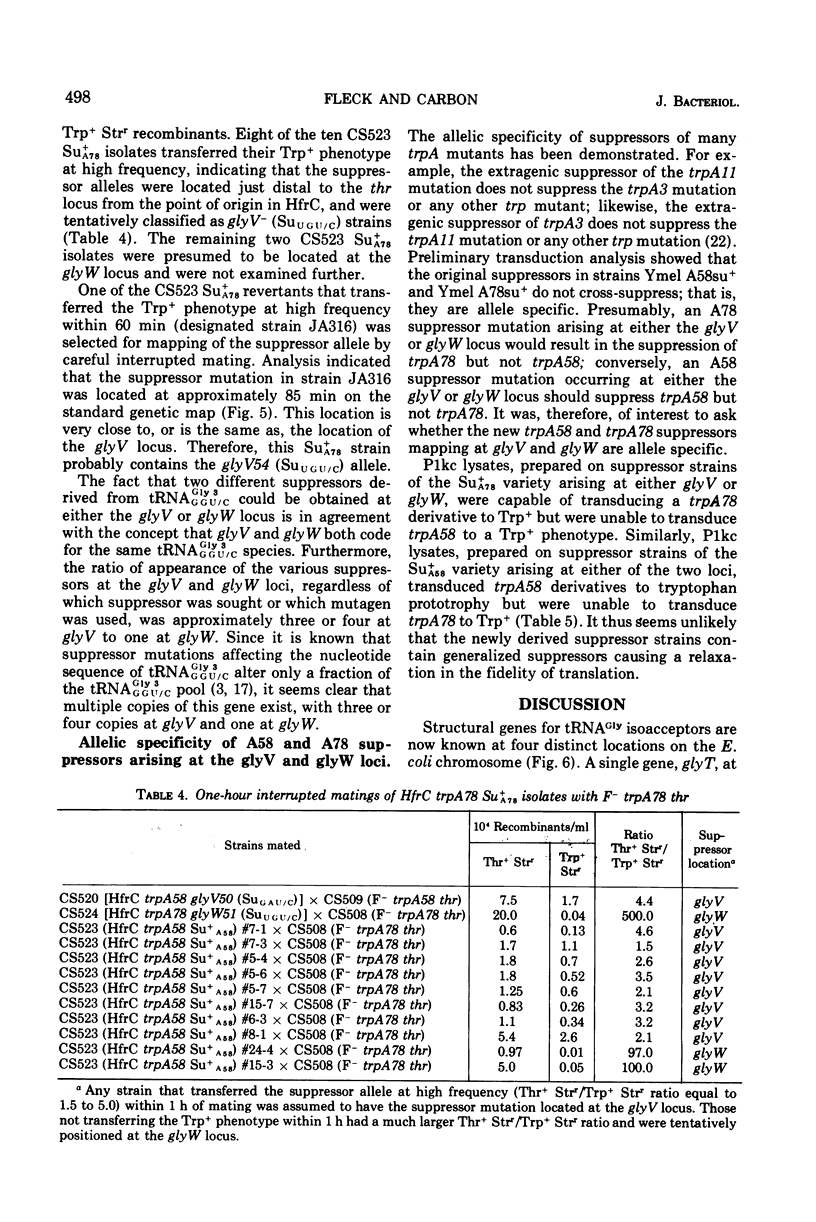
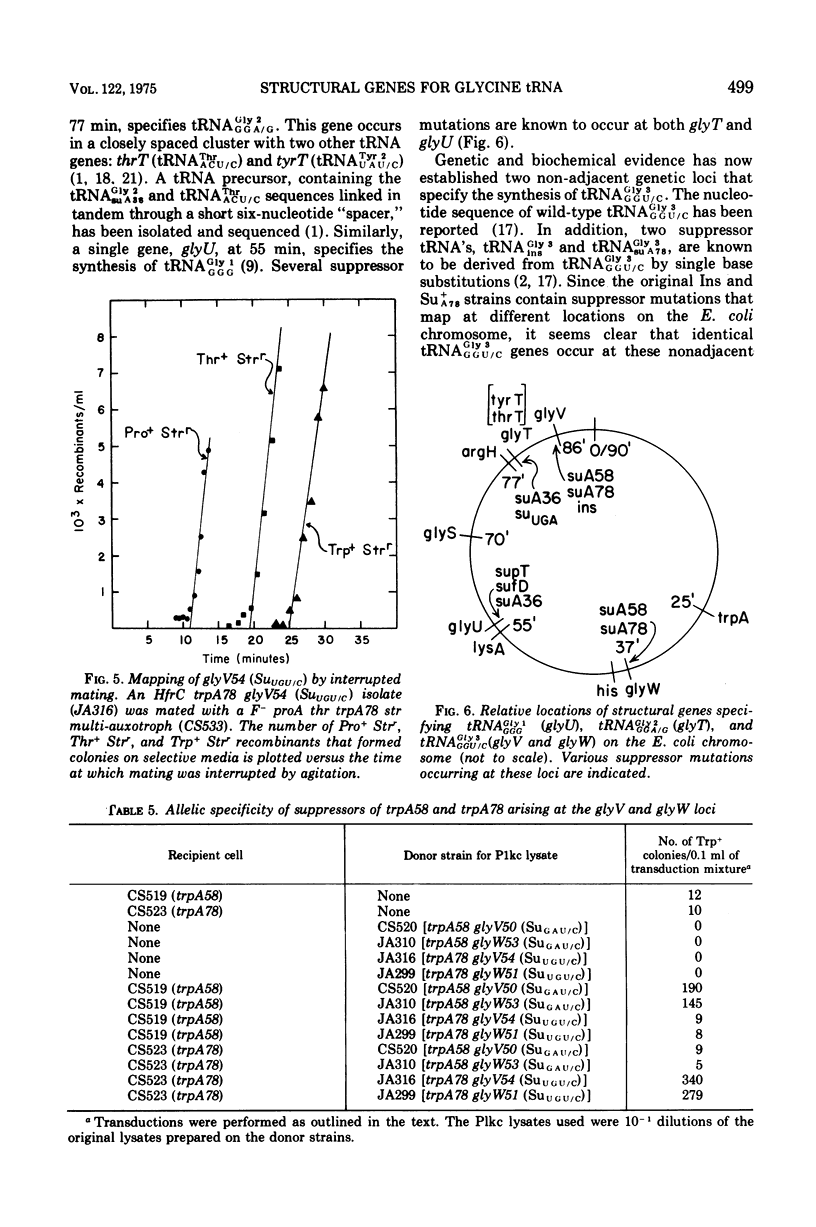
The study of suppressors of tryptophan synthase A protein missense mutations in Escherichia coli has led to the establishment of two nonadjacent genetic loci (gly V and gly W) specifying identical nucleotide sequences for a single isoaccepting species of glycine transfer ribonucleic acid (tRNA GLY 3 GGU/C). In one case, suppression of the missense mutation trpA78 was due to a mutation in a structural gene (gly W) for tRNA Gly 3 GGU/C. This mutation resulted in a base change in the anticodon and modification of an A residue adjacent to the 3' side of the anticodon, leading to the production of a tRNA Gly 3 UGU/C species. The resulting glyW51 (SU UGU/C) allele was mapped by interrupted mating and was located at approximately 37 min on the Escherichia coli genetic map. Other suppressor mutations affecting the primary sequence of tRNA Gly GGU/C and giving rise to the Ins and SU+A58 phenotypes were positioned at 86 min (glyV). Several independently arising missense suppressor mutations resulting in the SU+A78 phenotypes were isolated and mapped at these two genetic loci (glyV and glyW). The ratio of appearance of suppressor mutations at glyV and glyW suggests that there are three of four tRNAGly3 GGU/C structural gene copies at the glyV locus to one copy at the glyW locus. Structural genes for tRNA ly isoacceptors are now known at four distinct locations on the Escherichia coli chromosome: glyT (77 MIN), TRNA Gly 2 GGA/G; gly U (55 min), tRNAGly-1 minus; and gly V (86 MIN) AND GLYW (37 min), tRNAGly 3 GGU/C.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carbon J., Squires C., Hill C. W. Glycine transfer RNA of Escherichia coli. II. Impaired GGA-recognition in strains containing a genetically altered transfer RNA; reversal by a secondary suppressor mutation. J Mol Biol. 1970 Sep 28;52(3):571–584. doi: 10.1016/0022-2836(70)90420-1. [DOI] [PubMed] [Google Scholar]
- GUEST J. R., YANOFSKY C. MUTATIONALLY INDUCED AMINO ACID SUBSTITUTIONS IN A TRYPTIC PEPTIDE OF THE TRYPTOPHAN SYNTHETASE A PROTEIN. J Biol Chem. 1965 Feb;240:679–689. [PubMed] [Google Scholar]
- Glansdorff N., Sand G., Verhoef C. The dual genetic control of ornithine transcarbamylase synthesis in Escherichia coli K12. Mutat Res. 1967 Nov-Dec;4(6):743–751. doi: 10.1016/0027-5107(67)90083-8. [DOI] [PubMed] [Google Scholar]
- Guest J. R., Yanofsky C. Amino acid replacements associated with reversion and recombination within a coding unit. J Mol Biol. 1965 Jul;12(3):793–804. doi: 10.1016/s0022-2836(65)80328-x. [DOI] [PubMed] [Google Scholar]
- Hill C. W., Combriato G., Steinhart W., Riddle D. L., Carbon J. The nucleotide sequence of the GGG-specific glycine transfer ribonucleic acid of Escherichia coli and of Salmonella typhimurium. J Biol Chem. 1973 Jun 25;248(12):4252–4262. [PubMed] [Google Scholar]
- LOW B., WOOD T. H. A QUICK AND EFFICIENT METHOD FOR INTERRUPTION OF BACTERIAL CONJUGATION. Genet Res. 1965 Jul;6:300–303. doi: 10.1017/s001667230000416x. [DOI] [PubMed] [Google Scholar]
- Orias E., Gartner T. K., Lannan J. E., Betlach M. Close linkage between ochre and missense suppressors in Escherichia coli. J Bacteriol. 1972 Mar;109(3):1125–1133. doi: 10.1128/jb.109.3.1125-1133.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W., Carbon J. Molecular mechanism for missense suppression in E. coli. Nature. 1974 Aug 2;250(465):412–414. doi: 10.1038/250412a0. [DOI] [PubMed] [Google Scholar]
- Russell R. L., Abelson J. N., Landy A., Gefter M. L., Brenner S., Smith J. D. Duplicate genes for tyrosine transfer RNA in Escherichia coli. J Mol Biol. 1970 Jan 14;47(1):1–13. doi: 10.1016/0022-2836(70)90397-9. [DOI] [PubMed] [Google Scholar]
- Smith J. D. Gentics of transfer RNA. Annu Rev Genet. 1972;6:235–256. doi: 10.1146/annurev.ge.06.120172.001315. [DOI] [PubMed] [Google Scholar]
- Squires C., Carbon J., Hill C. W. Glycine transfer RNA of Escherichia coli. I. Structural genes for two glycine tRNA species. J Mol Biol. 1970 Sep 28;52(3):557–569. doi: 10.1016/0022-2836(70)90419-5. [DOI] [PubMed] [Google Scholar]
- Squires C., Konrad B., Kirschbaum J., Carbon J. Three adjacent transfer RNA genes in Escherichia coli. Proc Natl Acad Sci U S A. 1973 Feb;70(2):438–441. doi: 10.1073/pnas.70.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler J, Yanofsky C. Studies on a Series of Tryptophan-Independent Strains Derived from a Tryptophan-Requiring Mutant of Escherichia Coli. Genetics. 1959 Jan;44(1):105–123. doi: 10.1093/genetics/44.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Revised linkage map of Escherichia coli. Bacteriol Rev. 1967 Dec;31(4):332–353. doi: 10.1128/br.31.4.332-353.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Davidson N., Carbon J. Physical mapping of the transfer RNA genes on lambda-h80dglytsu+36. J Mol Biol. 1973 Jun 25;78(1):23–34. doi: 10.1016/0022-2836(73)90425-7. [DOI] [PubMed] [Google Scholar]
- YANOFSKY C., HELINSKI D. R., MALING B. D. The effects of mutation on the composition and properties of the A protein of Escherichia coli tryptohan synthetase. Cold Spring Harb Symp Quant Biol. 1961;26:11–24. doi: 10.1101/sqb.1961.026.01.006. [DOI] [PubMed] [Google Scholar]



