Abstract
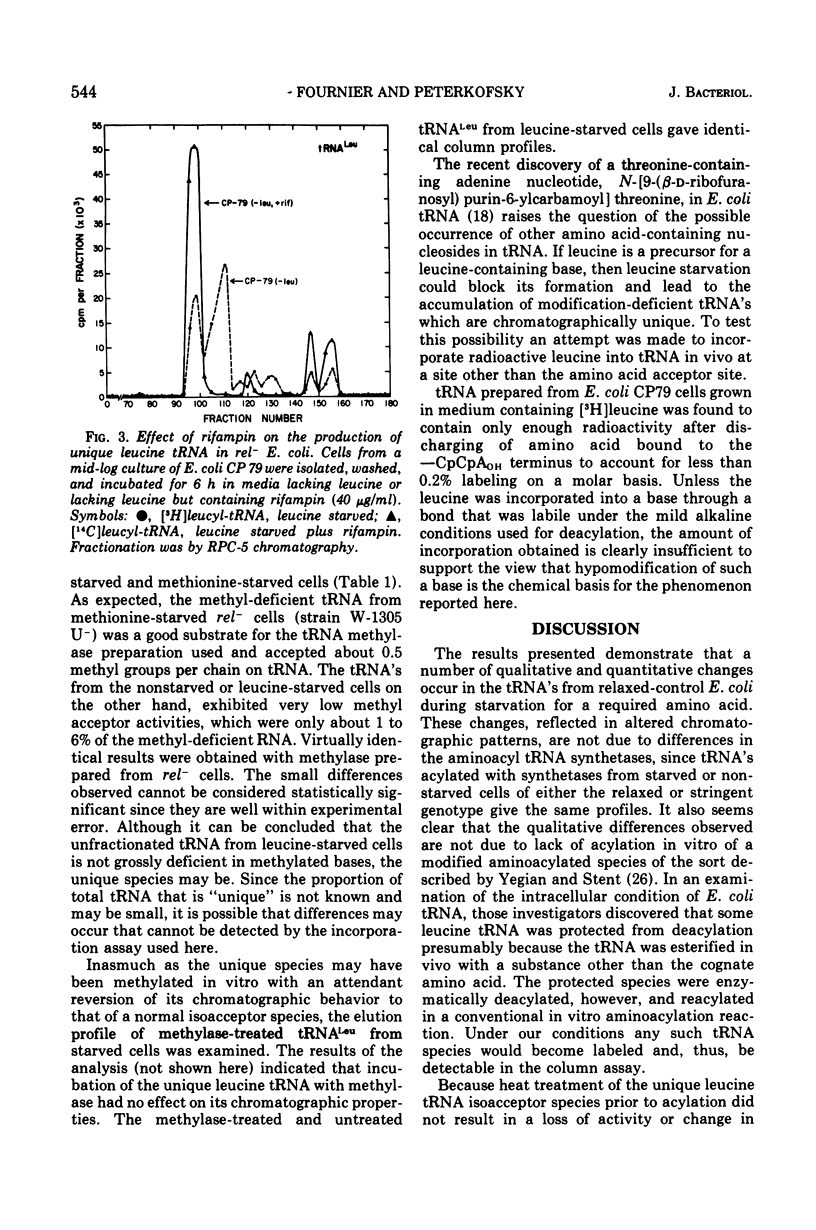
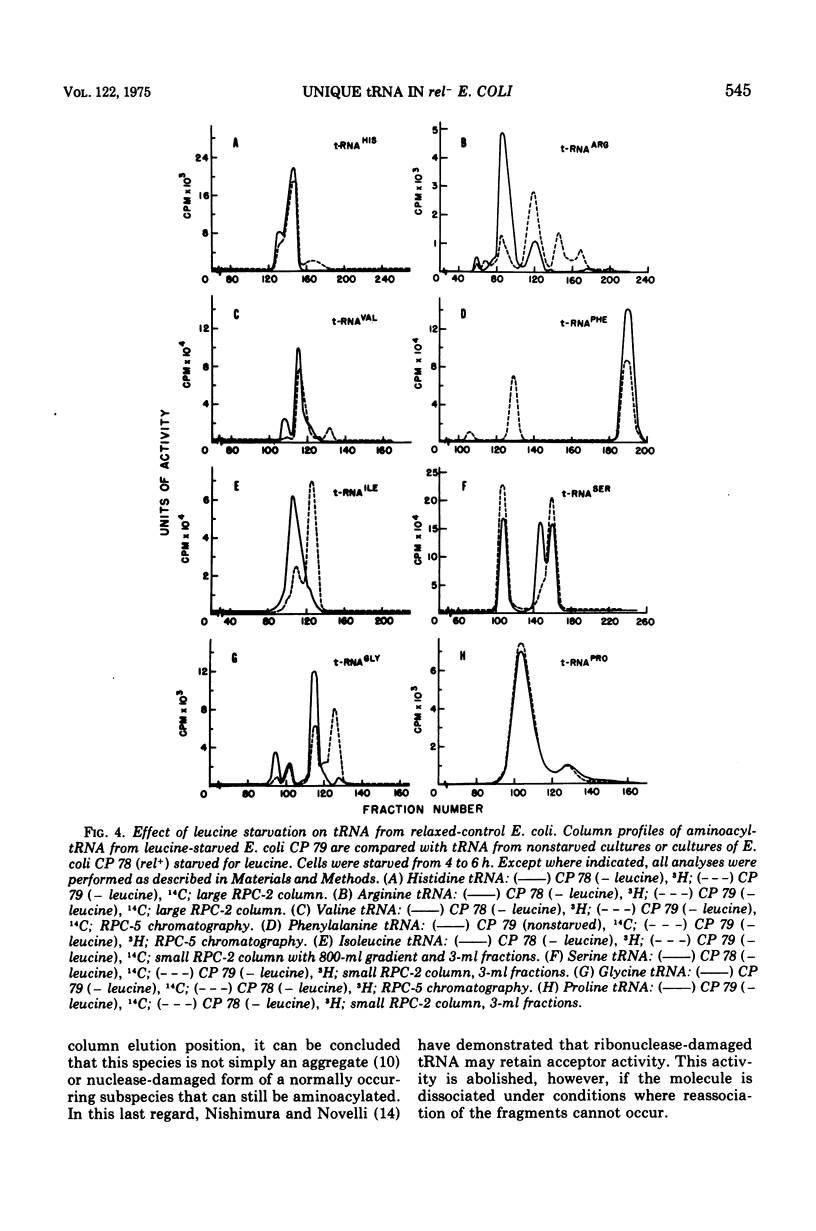
Examination of the transfer ribonucleic acid (tRNA) produced by starving, relaxed-control (rel minus) strains of Escherichia coli for required amino acids revealed the occurrence of a number of chromatographically unique subspecies. Leucine starvation results in the formation of new isoacceptor species of leucine-, histidine-, arginine-, valine-, and phenylalanine-specific tRNA and quantitative changes in the column profiles of serine, glycine, and isoleucine tRNA. Evidence that the unique tRNA species are synthesized de novo during amino acid starvation comes from the findings that the major unique leucine isoacceptor species is not formed in stringent control cells or in rel minus cells starved for uracil or treated with rifampin. Furthermore, heat treatment of the unique leucine tRNA does not alter its chromatographic behavior, indicating that the species is not an aggregate or nuclease-damaged form of a normal isoacceptor tRNA. The methyl acceptor activities of tRNA from leucine-starved and nonstarved rel+ or rel minus cells were found to be essentially the same. This result and the finding that the chromatographic behavior of the unique leucine-specific tRNA was not altered after treatment with tRNA methylase suggests that gross methyl deficiency is probably not the biochemical basis for the occurrence of the unique species.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman S., Robertson H. D. RNA precursor molecules and ribonucleases in E. coli. Mol Cell Biochem. 1973 May 11;1(1):83–93. doi: 10.1007/BF01659941. [DOI] [PubMed] [Google Scholar]
- Capra J. D., Peterkofsky A. Effect on in vitro methylation on the chromatographic and coding properties of methyl-deficient leucine transfer RNA. J Mol Biol. 1968 May 14;33(3):591–607. doi: 10.1016/0022-2836(68)90308-2. [DOI] [PubMed] [Google Scholar]
- Edlin G., Maaloe O. Synthesis and breakdown of messenger RNA without protein synthesis. J Mol Biol. 1966 Feb;15(2):428–434. doi: 10.1016/s0022-2836(66)80118-3. [DOI] [PubMed] [Google Scholar]
- FLEISSNER E., BOREK E. A new enzyme of RNA synthesis: RNA methylase. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1199–1203. doi: 10.1073/pnas.48.7.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M., Hedgcoth C. Levels of 5,6-dihydrouridine in relaxed and chloramphenicol transfer ribonucleic acid. Biochemistry. 1970 Jun 9;9(12):2513–2519. doi: 10.1021/bi00814a018. [DOI] [PubMed] [Google Scholar]
- LAZZARINI R. A., PETERKOFSKY A. THE CHARACTERIZATION OF A NEW SPECIES OF LEUCYL-SRNA FORMED DURING METHIONINE DEPRIVATION OF ESCHERICHIA COLI WITH RELAXED CONTROL. Proc Natl Acad Sci U S A. 1965 Mar;53:549–556. doi: 10.1073/pnas.53.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littauer U. Z., Inouye H. Regulation of tRNA. Annu Rev Biochem. 1973;42:439–470. doi: 10.1146/annurev.bi.42.070173.002255. [DOI] [PubMed] [Google Scholar]
- MANDEL L. R., BOREK E. Variability in the structure of ribonucleic acid. Biochem Biophys Res Commun. 1961 Jan 25;4:14–18. doi: 10.1016/0006-291x(61)90246-7. [DOI] [PubMed] [Google Scholar]
- NISHIMURA S., NOVELLI G. D. DISSOCIATION OF AMINO ACID ACCEPTOR FUNCTION OF SRNA FROM ITS TRANSFER FUNCTION. Proc Natl Acad Sci U S A. 1965 Jan;53:178–184. doi: 10.1073/pnas.53.1.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. L., Weiss J. F., Kelmers A. D. Improved separation of transfer RNA's on polychlorotrifuoroethylene-supported reversed-phase chromatography columns. Biochim Biophys Acta. 1971 Feb 11;228(3):770–774. doi: 10.1016/0005-2787(71)90748-9. [DOI] [PubMed] [Google Scholar]
- Rubin I. B., Kelmers A. D., Goldstein G. The determination of transfer ribonucleic acid by aminoacylation. I. Leucine and phenylalanine transfer ribonucleic acid from E. coli B. Anal Biochem. 1967 Sep;20(3):533–544. doi: 10.1016/0003-2697(67)90298-9. [DOI] [PubMed] [Google Scholar]
- Ryan A. M., Borek E. The relaxed control phenomenon. Prog Nucleic Acid Res Mol Biol. 1971;11:193–228. doi: 10.1016/s0079-6603(08)60328-1. [DOI] [PubMed] [Google Scholar]
- Schweizer M. P., Chheda G. B., Baczynskyj L., Hall R. H. Aminoacyl nucleosides. VII. N-(Purin-6-ylcarbamoyl)threonine. A new component of transfer ribonucleic acid. Biochemistry. 1969 Aug;8(8):3283–3289. doi: 10.1021/bi00836a023. [DOI] [PubMed] [Google Scholar]
- Sueoka N., Kano-Sueoka T. Transfer RNA and cell differentiation. Prog Nucleic Acid Res Mol Biol. 1970;10:23–55. doi: 10.1016/s0079-6603(08)60560-7. [DOI] [PubMed] [Google Scholar]
- Söll D. Enzymatic modification of transfer RNA. Science. 1971 Jul 23;173(3994):293–299. doi: 10.1126/science.173.3994.293. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Waters L. C. Altered chromatographic properties of tRNA from chloramphenicol-treated Escherichia coli. Biochem Biophys Res Commun. 1969 Oct 8;37(2):296–304. doi: 10.1016/0006-291x(69)90734-7. [DOI] [PubMed] [Google Scholar]
- Waters L. C., Shugart L., Yang W. K., Best A. N. Some physical and biological properties of 4-thiouridine- and dihydrouridine-deficient tRNA from chloramphenicol-treated Escherichia coli. Arch Biochem Biophys. 1973 Jun;156(2):780–793. doi: 10.1016/0003-9861(73)90332-9. [DOI] [PubMed] [Google Scholar]
- Weiss J. F., Kelmers A. D. A new chromatographic system for increased resolution of transfer ribonucleic acids. Biochemistry. 1967 Aug;6(8):2507–2513. doi: 10.1021/bi00860a030. [DOI] [PubMed] [Google Scholar]
- Williams L. S., Freundlich M. Role of valine transfer RNA in control of RNA synthesis in Escherichia coli. Biochim Biophys Acta. 1969 Apr 22;179(2):515–517. doi: 10.1016/0005-2787(69)90064-1. [DOI] [PubMed] [Google Scholar]
- Yegian C. D., Stent G. S. An unusual condition of leucine transfer RNA appearing during leucine starvation of Escherichia coli. J Mol Biol. 1969 Jan 14;39(1):45–58. doi: 10.1016/0022-2836(69)90332-5. [DOI] [PubMed] [Google Scholar]
- Zachau H. G. Transfer ribonucleic acids. Angew Chem Int Ed Engl. 1969 Oct;8(10):711–727. doi: 10.1002/anie.196907111. [DOI] [PubMed] [Google Scholar]


