Abstract
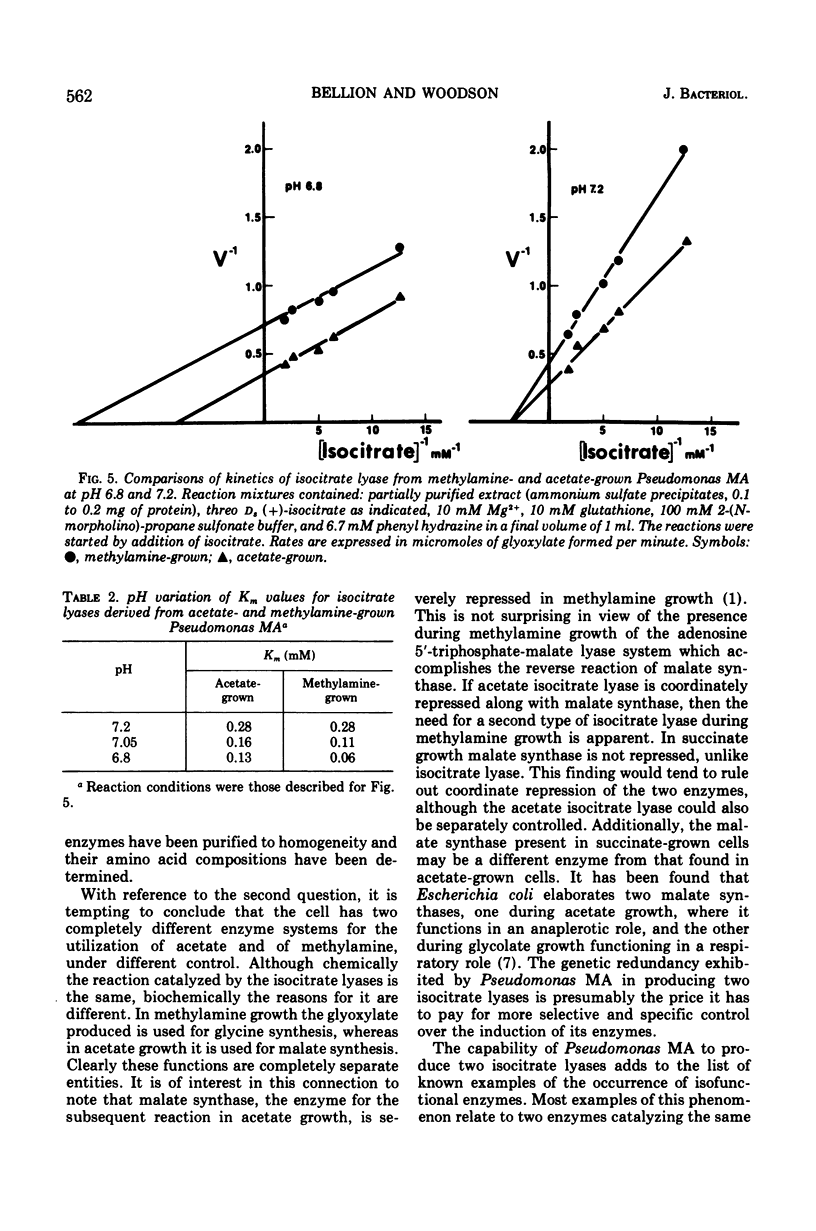
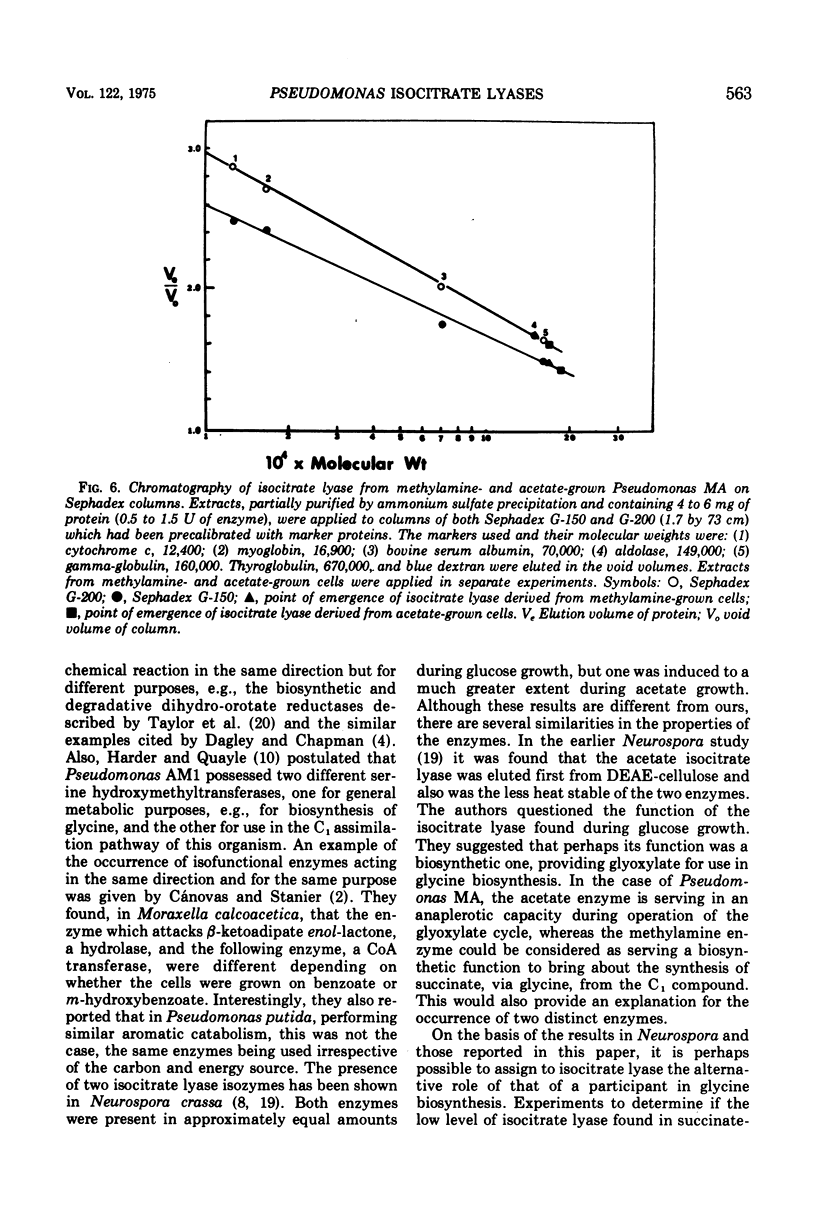
The isocitrate lyases of acetate- and methylamine-grown Pseudomonas MA (Shaw strain) were studied. They were shown to be different by a variety of physical criteria including chromatographic elution patterns, heat inactivation kinetics, pH variation of Km values, and migration on polyacrylamide gels. The implications and significance of the existence of two enzymes in relation to the role of isocitrate lyase in methylamine utilization is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellion E., Hersh L. B. Methylamine metabolism in a pseudomonas species. Arch Biochem Biophys. 1972 Nov;153(1):368–374. doi: 10.1016/0003-9861(72)90457-2. [DOI] [PubMed] [Google Scholar]
- Cánovas J. L., Stanier R. Y. Regulation of the enzymes of the beta-ketoadipate pathway in Moraxella calcoacetica. 1. General aspects. Eur J Biochem. 1967 May;1(3):289–300. doi: 10.1007/978-3-662-25813-2_40. [DOI] [PubMed] [Google Scholar]
- FALMAGNE P., VANDERWINKEL E., WIAME J. M. MISE EN EVIDENCE DE DEUX MALATE SYNTHASES CHEZ ESCHERICHIA COLI. Biochim Biophys Acta. 1965 May 18;99:246–258. [PubMed] [Google Scholar]
- Flavell R. B., Woodward D. O. Metabolic role, regulation of synthesis, cellular localization, and genetic control of the glyoxylate cycle enzymes in Neurospora crassa. J Bacteriol. 1971 Jan;105(1):200–210. doi: 10.1128/jb.105.1.200-210.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder W., Quayle J. R. The biosynthesis of serine and glycine in Pseudomonas AM1 with special reference to growth on carbon sources other than C1 compounds. Biochem J. 1971 Mar;121(5):753–762. doi: 10.1042/bj1210753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh L. B., Bellion E. Malate cleavage reaction in Pseudomonas species, (Shaw strain MA). Biochem Biophys Res Commun. 1972 Aug 7;48(3):712–719. doi: 10.1016/0006-291x(72)90407-x. [DOI] [PubMed] [Google Scholar]
- Hersh L. B. Malate adenosine triphosphate lyase. Separation of the reaction into a malate thiokinase and malyl coenzyme A lyase. J Biol Chem. 1973 Nov 10;248(21):7295–7303. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McFadden B. A., Rao G. R., Cohen A. L., Roche T. E. Isocitrate lyase from Pseudomonas indigofera. V. Subunits and terminal residues and the relation to catalytic activity. Biochemistry. 1968 Oct;7(10):3574–3582. doi: 10.1021/bi00850a035. [DOI] [PubMed] [Google Scholar]
- Reeves H. C., Volk M. J. Determination of isocitrate lyase activity in polyacrylamide gels. Anal Biochem. 1972 Aug;48(2):437–441. doi: 10.1016/0003-2697(72)90096-6. [DOI] [PubMed] [Google Scholar]
- Shaw W. V., Tsai L., Stadtman E. R. The enzymatic synthesis of N-methylglutamic acid. J Biol Chem. 1966 Feb 25;241(4):935–945. [PubMed] [Google Scholar]
- Sjogren R. E., Romano A. H. Evidence for multiple forms of isocitrate lyase in Neurospora crassa. J Bacteriol. 1967 May;93(5):1638–1643. doi: 10.1128/jb.93.5.1638-1643.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W. H., Taylor M. L., Eames D. F. Two functionally different dihydroorotic dehydrogenases in bacteria. J Bacteriol. 1966 Jun;91(6):2251–2256. doi: 10.1128/jb.91.6.2251-2256.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]