Abstract
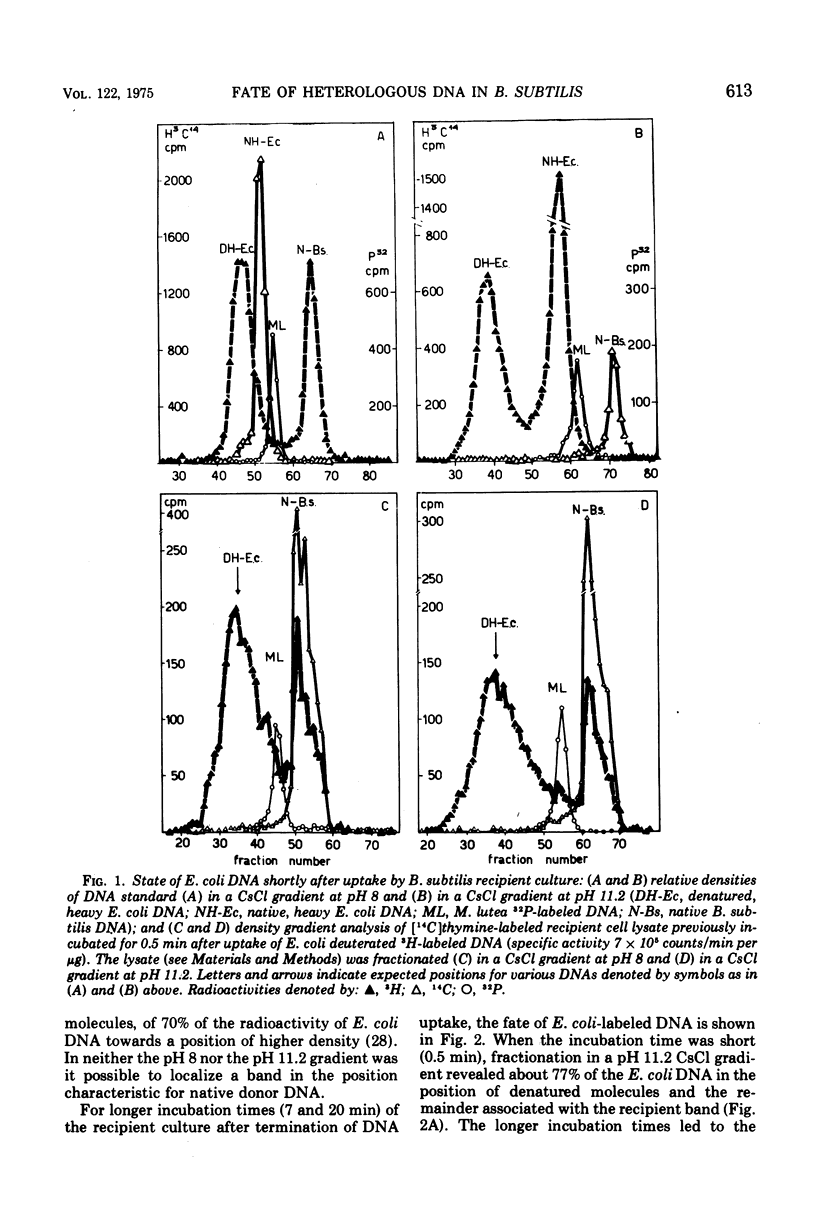
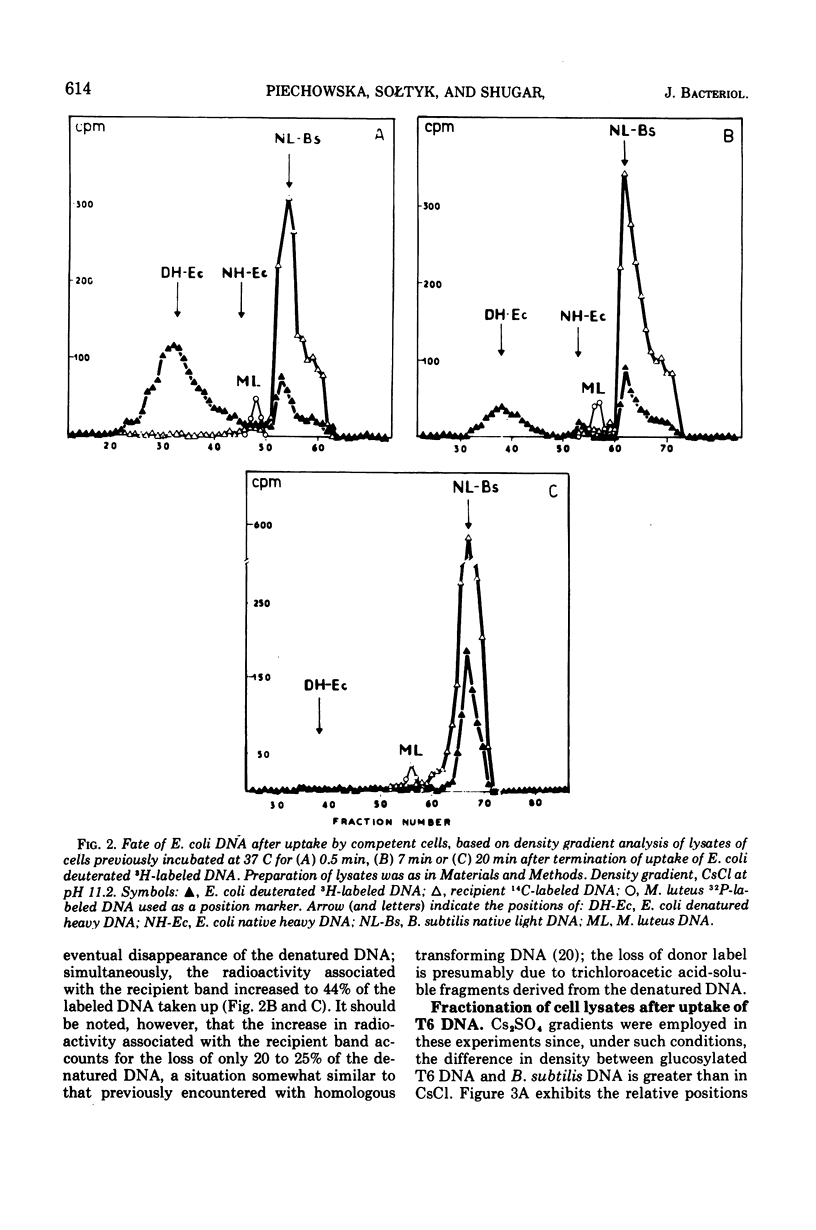
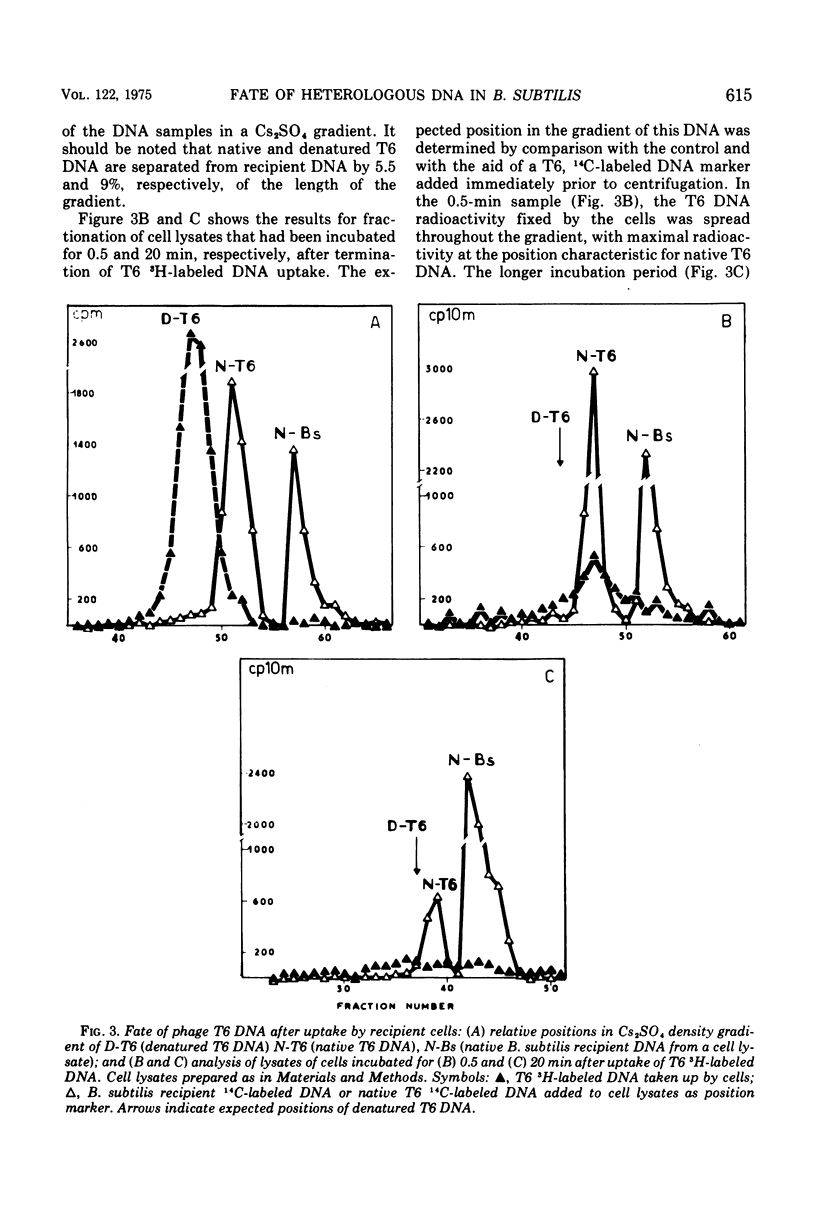
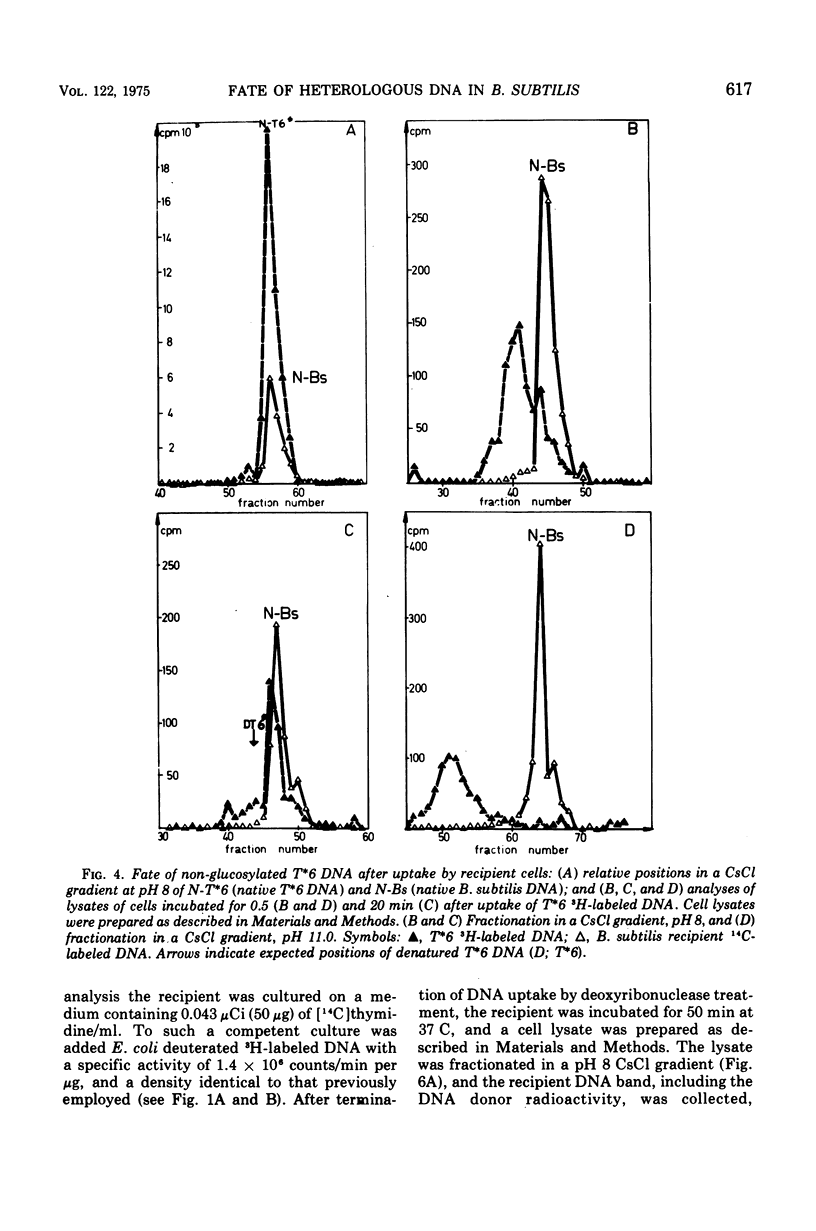
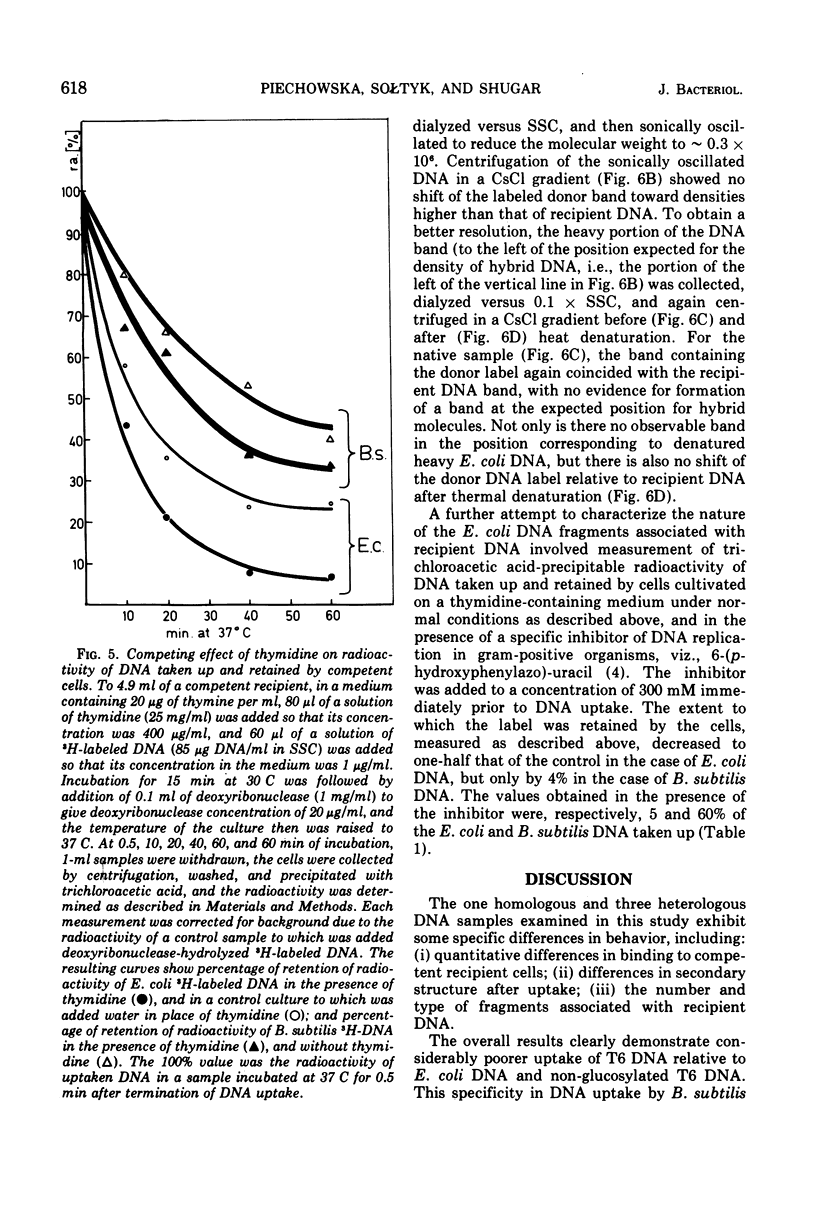
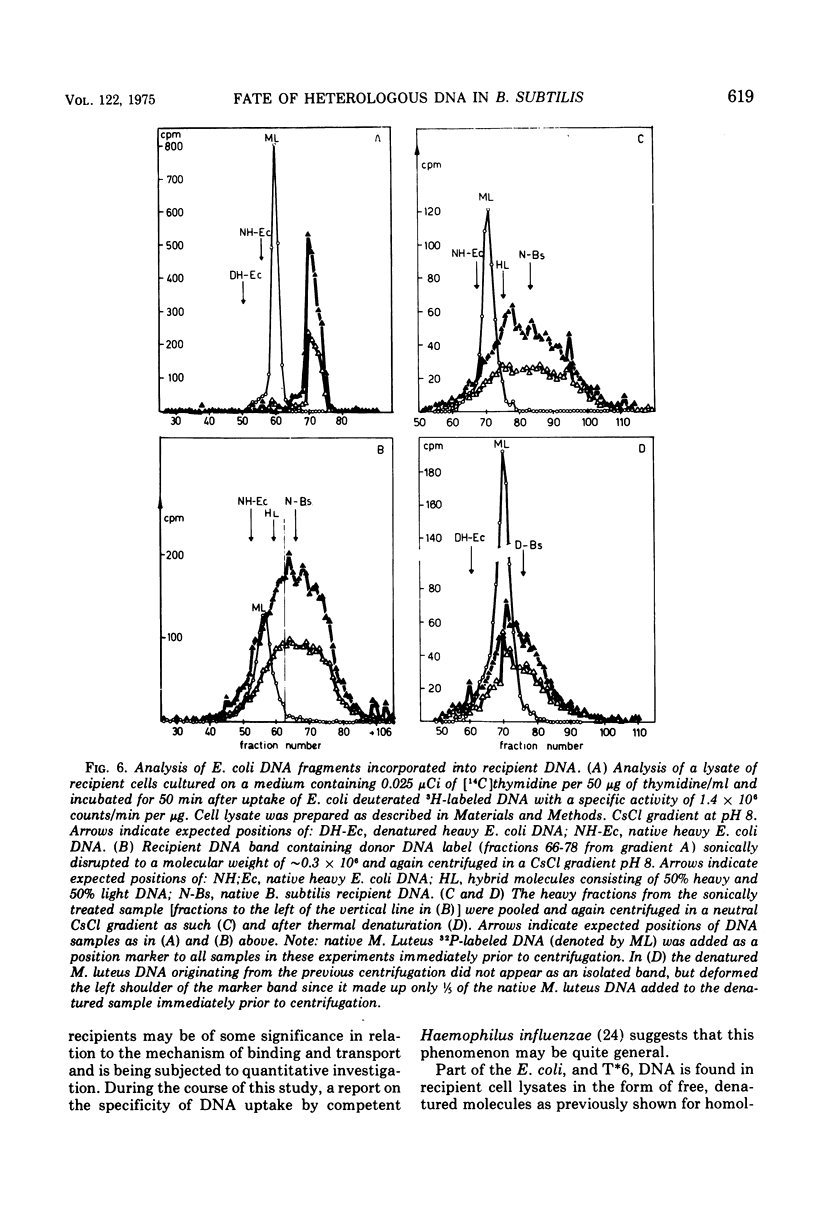
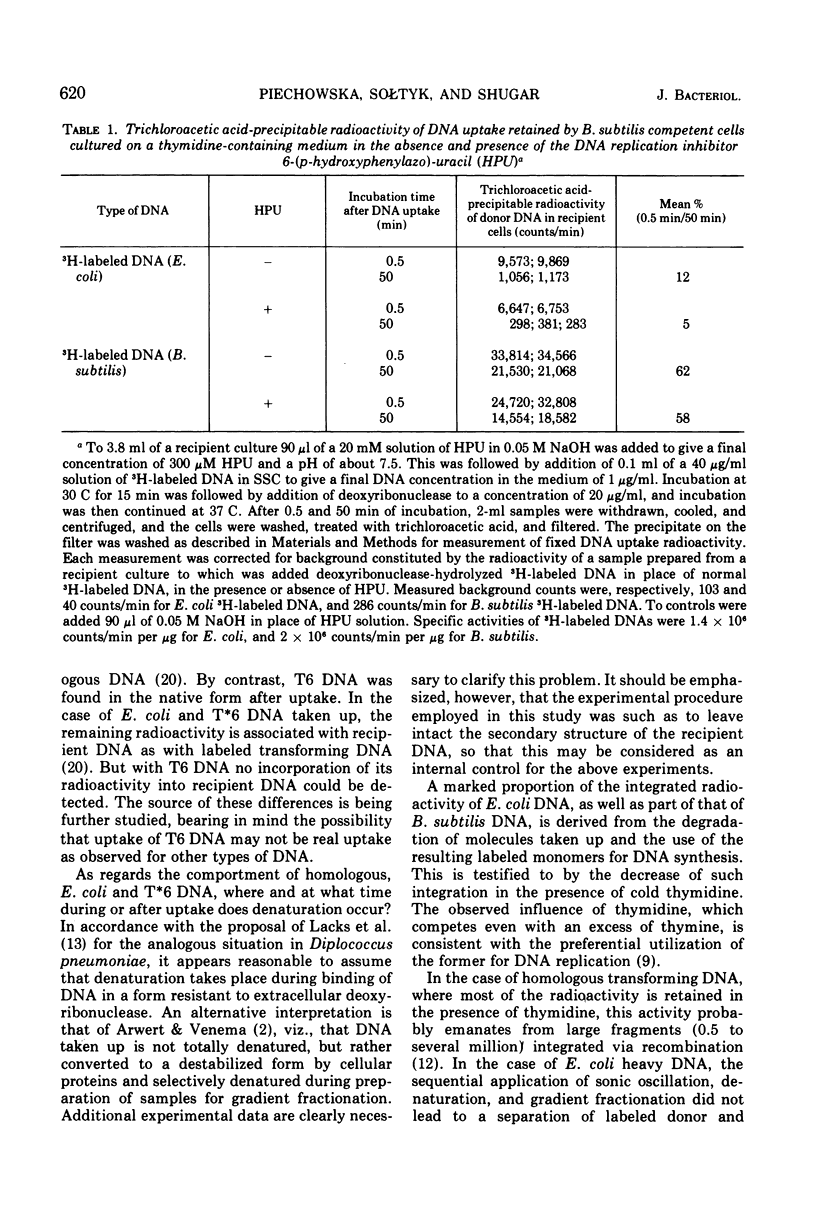
CsCl density gradient fractionation of cell lysates was employed to follow the fate of Escherichia coli, phage T6, and non-glucosylated phage T6 deoxyribonucleic acid (DNA) after uptake by competent cells of Bacillus subtilis 168 thy minus trp minus. Shortly after uptake, most of the radioactive Escherichia coli or non-glucosylated T6 DNA was found in the denatured form; the remainder of the label was associated with recipient DNA. Incubation of the cells after DNA uptake led to the disappearance of denatured donor DNA and to an increase in the amount of donor label associated with recipient DNA. These findings are analogous to those previously reported with homologous DNA. By contrast, T6 DNA, which is poorly taken up, appeared in the native form shortly after uptake and was degraded on subsequent incubation. The nature of the heterologous DNA fragments associated with recipient DNA was investigated with Escherichia coli 2-H and 3-H-labeled DNA. Association of radioactivity with recipient DNA decreased to one-fourth in the presence of excess thymidine; residual radioactivity could not be separated from recipient DNA by shearing (sonic oscillation) and/or denaturation, but was reduced by one-half in the presence of a DNA replication inhibitor. Residual radioactivity associated with donor DNA under these conditions was about 5% of that originally taken up. Excess thymidine, but not the DNA replication inhibitor, also decreased association of homologous DNA label with recipient DNA; but, even in the presence of both of these, the decrease amounted to only 60%. It is concluded that most, or all, of the Escherichia coli DNA label taken up is associated with recipient DNA in the form of mononucleotides via DNA replication.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. H. Growth Requirements of Virus-Resistant Mutants of Escherichia Coli Strain "B". Proc Natl Acad Sci U S A. 1946 May;32(5):120–128. doi: 10.1073/pnas.32.5.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arwert F., Venema G. Transformation in Bacillus subtilis. Fate of newly introduced transforming DNA. Mol Gen Genet. 1973;123(2):185–198. doi: 10.1007/BF00267334. [DOI] [PubMed] [Google Scholar]
- BODMER W. F., GANESAN A. T. BIOCHEMICAL AND GENETIC STUDIES OF INTEGRATION AND RECOMBINATION IN BACILLUS SUBTILIS TRANSFORMATION. Genetics. 1964 Oct;50:717–738. doi: 10.1093/genetics/50.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. C. Inhibition of bacterial DNA replication by 6-(p-hydroxyphenylazo)-uracil: differential effect on repair and semi-conservative synthesis in Bacillus subtilis. J Mol Biol. 1971 Jul 14;59(1):1–16. doi: 10.1016/0022-2836(71)90409-8. [DOI] [PubMed] [Google Scholar]
- CRAWFORD L., DULBECCO R., FRIED M., MONTAGNIER L., STOKER M. CELL TRANSFORMATION BY DIFFERENT FORMS OF POLYOMA VIRUS DNA. Proc Natl Acad Sci U S A. 1964 Jul;52:148–152. doi: 10.1073/pnas.52.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidoff-Abelson R., Dubnau D. Kinetic analysis of the products of donor deoxyribonucleate in transformed cells of Bacillus subtilis. J Bacteriol. 1973 Oct;116(1):154–162. doi: 10.1128/jb.116.1.154-162.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Cirigliano C. Fate of transforming DNA following uptake by competent Bacillus subtilis. Formation and properties of products isolated from transformed cells which are derived entirely from donor DNA. J Mol Biol. 1972 Feb 28;64(1):9–29. doi: 10.1016/0022-2836(72)90318-x. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Cirigliano C. Fate of transforming deoxyribonucleic acid after uptake by competent Bacillus subtilis: nonrequirement of deoxyribonucleic acid replication for uptake and integration of transforming deoxyribonucleic acid. J Bacteriol. 1973 Mar;113(3):1512–1514. doi: 10.1128/jb.113.3.1512-1514.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HATTMAN S., FUKASAWA T. HOST-INDUCED MODIFICATION OF T-EVEN PHAGES DUE TO DEFECTIVE GLUCOSYLATION OF THEIR DNA. Proc Natl Acad Sci U S A. 1963 Aug;50:297–300. doi: 10.1073/pnas.50.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris W. J. The occurrence of two types of synthesis of deoxyribonucleic acid during normal growth in Bacillus subtilis. Biochem J. 1973 Oct;135(2):315–325. doi: 10.1042/bj1350315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss R. D., Gabor M. Bacterial transformation, with special reference to recombination process. Annu Rev Genet. 1970;4:193–224. doi: 10.1146/annurev.ge.04.120170.001205. [DOI] [PubMed] [Google Scholar]
- Leff J., Beardsley R. E. Action tumorigène de l'acide nucleique d'un bactériophage présent dans les cultures de tissu tumoral de tournesol (Helianthus annus) C R Acad Sci Hebd Seances Acad Sci D. 1970 May 20;270(20):2505–2507. [PubMed] [Google Scholar]
- Losick R., Shorenstein R. G., Sonenshein A. L. Structural alteration of RNA polymerase during sporulation. Nature. 1970 Aug 29;227(5261):910–913. doi: 10.1038/227910a0. [DOI] [PubMed] [Google Scholar]
- McCarthy C., Nester E. W. Macromolecular synthesis in newly transformed cells of Bacillus subtilis. J Bacteriol. 1967 Jul;94(1):131–140. doi: 10.1128/jb.94.1.131-140.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergeay M. Mutagenic effects of Streptomyces coelicolor DNA detected after streptomycin treatment of competent cultures of Bacillus subtilis. Mol Gen Genet. 1972;119(1):89–92. doi: 10.1007/BF00270448. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Geier M. R., Petricciani J. C. Bacterial virus gene expression in human cells. Nature. 1971 Oct 8;233(5319):398–400. doi: 10.1038/233398a0. [DOI] [PubMed] [Google Scholar]
- Piechowska M., Fox M. S. Fate of transforming deoxyribonucleate in Bacillus subtilis. J Bacteriol. 1971 Nov;108(2):680–689. doi: 10.1128/jb.108.2.680-689.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechowska M., Shugar D. Inhibitory and lethal effects of DNA on transformable streptococci. Biochem Biophys Res Commun. 1967 Feb 8;26(3):290–295. doi: 10.1016/0006-291x(67)90120-9. [DOI] [PubMed] [Google Scholar]
- Rosenthal P. N., Fox M. S. Effects of disintegration of incorporated 3H and 32P on the physical and biological properties of DNA. J Mol Biol. 1970 Dec 28;54(3):441–463. doi: 10.1016/0022-2836(70)90120-8. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Scocca J. J., Poland R. L., Zoon K. C. Specificity in deoxyribonucleic acid uptake by transformable Haemophilus influenzae. J Bacteriol. 1974 May;118(2):369–373. doi: 10.1128/jb.118.2.369-373.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein E., Mehta B. M. Fate of Bacillus subtilis transforming deoxyribonucleic acid incorporated into transformable Diplococcus pneumoniae. Biochemistry. 1971 Feb 16;10(4):683–691. doi: 10.1021/bi00780a021. [DOI] [PubMed] [Google Scholar]
- VINOGRAD J., MORRIS J., DAVIDSON N., DOVE W. F., Jr The bouyant behavior of viral and bacterial DNA in alkaline CsCl. Proc Natl Acad Sci U S A. 1963 Jan 15;49:12–17. doi: 10.1073/pnas.49.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]


