Abstract
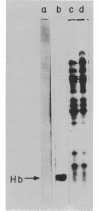
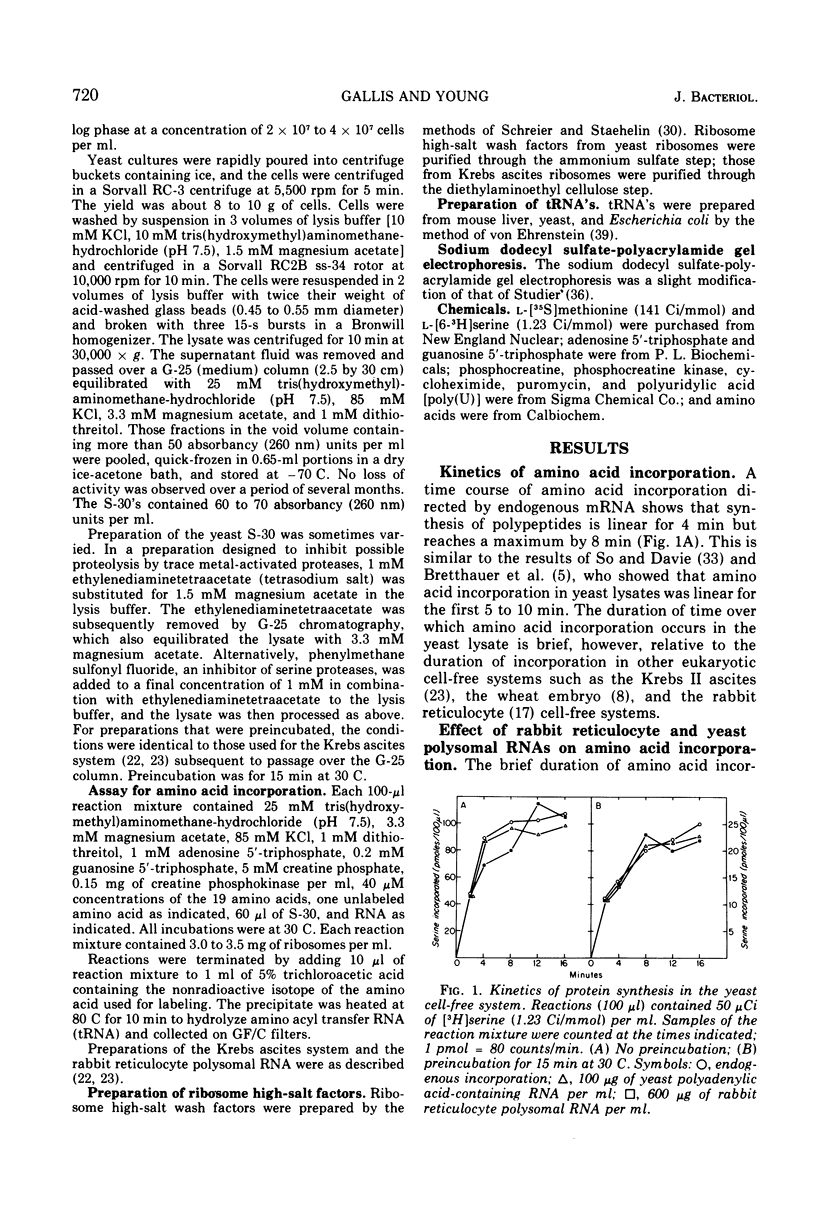
An in vitro protein-synthesizing system from the yeast Saccharomyces cerevisiae has been made by a modification of the procedure for preparation of the Krebs ascites system. The protein synthetic activity is directed by endogenous messenger. Amino acid incorporation occurs over a broad range of magnesium and potassium concentration, being maximal at 6 and 85 mM, respcetively. The activity of this in vitro system is due to the elongation of polypeptides whose synthesis was initiated in vivo. The cell extract does not initiate synthesis with endogenous messenger ribonucleic acid (RNA), since 1 muM pactamycin, which blocks initiation on prokaryotic or eukaryotic ribosomes invitro, fails to decrease amino acid incorporation. Ten micromolar cycloheximide, however, inhibits incorporation by 87%. Moreover, this system is not stimulated by rabbit reticulocyte polysomal RNA, which directs the synthesis of hemoglobin in extracts of Krebs ascites cells. The translation of this messenger is not masked by high endogenous incorporation, because autoradiography of sodium dodecyl sulfate-polyacrylamide gels containing [35-S]methionine-labeled products shows that no hemoglobin is made. Preincubation of this system, which reduces the high endogenous incorporation by 80%, does not increase its capacity to be stimulated by either rabbit reticulocyte RNA or yeast polyriboadenylic acid-containing RNA. Polyuridylic acid, however, does stimulate polyphenylalanine incorporation. The failure of the yeast lysate to be stimulated by or to translate added natural messenger RNA, its insensitivity to low levels of pactamycin but inhibition by cycloheximide, and its relatively high magnesium optimum (the same as that for polyuridylic acid) suggest that it elongates but does not initiate polypeptide chains.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson S. D., Herbert E., Godchaux W. Factors affecting the rate of protein synthesis in lysate systems from reticulocytes. Arch Biochem Biophys. 1968 May;125(2):671–683. doi: 10.1016/0003-9861(68)90625-5. [DOI] [PubMed] [Google Scholar]
- Aviv H., Boime I., Leder P. Protein synthesis directed by encephalomyocarditis virus RNA: properties of a transfer RNA-dependent system. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2303–2307. doi: 10.1073/pnas.68.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRETTHAUER R. K., MARCUS L., CHALOUPKA J., HALVORSON H. O., BOCK R. M. AMINO ACID INCORPORATION INTO PROTEIN BY CELL-FREE EXTRACTS OF YEAST. Biochemistry. 1963 Sep-Oct;2:1079–1084. doi: 10.1021/bi00905a029. [DOI] [PubMed] [Google Scholar]
- Benveniste K., Wilczek J., Stern R. Translation of collagen mRNA from chick embryo calvaria in a cell-free system derived from Krebs II ascites cells. Nature. 1973 Nov 30;246(5431):303–305. doi: 10.1038/246303b0. [DOI] [PubMed] [Google Scholar]
- Blobel G., Sabatini D. Dissociation of mammalian polyribosomes into subunits by puromycin. Proc Natl Acad Sci U S A. 1971 Feb;68(2):390–394. doi: 10.1073/pnas.68.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. E., Kolb A. J., Stanley W. M., Jr A general procedure for the preparation of highly active eukaryotic ribosomes and ribosomal subunits. Methods Enzymol. 1974;30:368–387. doi: 10.1016/0076-6879(74)30039-0. [DOI] [PubMed] [Google Scholar]
- Cohen L. B., Herner A. E., Goldberg I. H. Inhibition by pactamycin of the initiation of protein synthesis. Binding of N-acetylphenylalanyl transfer ribonucleic acid and polyuridylic acid to ribosomes. Biochemistry. 1969 Apr;8(4):1312–1326. doi: 10.1021/bi00832a004. [DOI] [PubMed] [Google Scholar]
- Gallis B. M., McDonnell J. P., Hopper J. E., Young E. T. Translation of poly(riboadenylic acid)-enriched messenger RNAs from the yeast, Saccharomyces cerevisiae, in heterologous cell-free systems. Biochemistry. 1975 Mar 11;14(5):1038–1046. doi: 10.1021/bi00676a024. [DOI] [PubMed] [Google Scholar]
- Godchaux W., 3rd, Adamson S. D., Herbert E. Effects of cycloheximide on polyribosome function in reticulocytes. J Mol Biol. 1967 Jul 14;27(1):57–72. doi: 10.1016/0022-2836(67)90351-8. [DOI] [PubMed] [Google Scholar]
- Gottlieb M., Lubsen N. H., Davis B. D. Preparation and assay of ribosome dissociation factors from Escherichia coli and rabbit reticulocytes and the assay for free ribosomes. Methods Enzymol. 1974;30:87–94. doi: 10.1016/0076-6879(74)30012-2. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H. Biochemical genetics of yeast. Annu Rev Genet. 1970;4:373–396. doi: 10.1146/annurev.ge.04.120170.002105. [DOI] [PubMed] [Google Scholar]
- Kaempfer R., Kaufman J. Translational control of hemoglobin synthesis by an initiation factor required for recycling of ribosomes and for their binding to messenger RNA. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3317–3321. doi: 10.1073/pnas.69.11.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappen L. S., Suzuki H., Goldberg I. H. Inhibition of reticulocyte peptide-chain initiation by pactamycin: accumulation of inactive ribosomal initiation complexes. Proc Natl Acad Sci U S A. 1973 Jan;70(1):22–26. doi: 10.1073/pnas.70.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebleu B., Nudel U., Falcoff E., Prives C., Revel M. A comparison of the translation of Mengo virus RNA and globin mRNA in krebs ascites cell-free extracts. FEBS Lett. 1972 Sep 1;25(1):97–103. doi: 10.1016/0014-5793(72)80463-0. [DOI] [PubMed] [Google Scholar]
- Lockard R. E., Lingrel J. B. Mouse hemoglobin messenger ribonucleic acid. Translational capacities of rabbit and duck reticulocyte cell-free systems programmed with mouse 9 S ribonucleic acid. J Biol Chem. 1972 Jul 10;247(13):4174–4179. [PubMed] [Google Scholar]
- Lockard R. E., Lingrel J. B. The synthesis of mouse hemoglobin beta-chains in a rabbit reticulocyte cell-free system programmed with mouse reticulocyte 9S RNA. Biochem Biophys Res Commun. 1969 Oct 8;37(2):204–212. doi: 10.1016/0006-291x(69)90720-7. [DOI] [PubMed] [Google Scholar]
- Lockard R. E., Lingrel J. B. Translation of mammalian globin messenger RNAs in an avian reticulocyte cell-free system. Biochim Biophys Acta. 1973 Feb 23;299(1):148–152. doi: 10.1016/0005-2787(73)90406-1. [DOI] [PubMed] [Google Scholar]
- Lodish H. G., Housman D., Jacobsen M. Initiation of hemoglobin synthesis. Specific inhibition by antibiotics and bacteriophage ribonucleic acid. Biochemistry. 1971 Jun 8;10(12):2348–2356. doi: 10.1021/bi00788a027. [DOI] [PubMed] [Google Scholar]
- Lubsen N. H., Davis B. D. A ribosome dissociation factor from rabbit reticulocytes (fluoride-E. coli ribosomes-initiation factors). Proc Natl Acad Sci U S A. 1972 Feb;69(2):353–357. doi: 10.1073/pnas.69.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald J. S., Goldberg I. H. An effect of pactamycin on the initiation of protein synthesis in reticulocytes. Biochem Biophys Res Commun. 1970 Oct 9;41(1):1–8. doi: 10.1016/0006-291x(70)90460-2. [DOI] [PubMed] [Google Scholar]
- Mathews M. B. Further studies on the translation of globin mRNA and encephalomyocarditis virus RNA in a cell-free system from Krebs II ascites cells. Biochim Biophys Acta. 1972 Jun 22;272(1):108–118. doi: 10.1016/0005-2787(72)90038-x. [DOI] [PubMed] [Google Scholar]
- Mathews M. B., Pragnell I. B., Osborn M., Arnstein H. R. Stimulation by reticulocyte initiation factors of protein synthesis in a cell-free system from Krebs II ascites cells. Biochim Biophys Acta. 1972 Nov 16;287(1):113–123. doi: 10.1016/0005-2787(72)90335-8. [DOI] [PubMed] [Google Scholar]
- Mathews M., Korner A. Mammalian cell-free protein synthesis directed by viral ribonucleic acid. Eur J Biochem. 1970 Dec;17(2):328–338. doi: 10.1111/j.1432-1033.1970.tb01170.x. [DOI] [PubMed] [Google Scholar]
- McLaughlin C. S., Warner J. R., Edmonds M., Nakazato H., Vaughan M. H. Polyadenylic acid sequences in yeast messenger ribonucleic acid. J Biol Chem. 1973 Feb 25;248(4):1466–1471. [PubMed] [Google Scholar]
- Metafora S., Terada M., Dow L. W., Marks P. A., Bank A. Increased efficiency of exogenous messenger RNA translation in a Krebs ascites cell lysate. Proc Natl Acad Sci U S A. 1972 May;69(5):1299–1303. doi: 10.1073/pnas.69.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudel U., Lebleu B., Revel M. Discrimination between messenger ribonucleic acids by a mammalian translation initiation factor. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2139–2144. doi: 10.1073/pnas.70.7.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SO A. G., DAVIE E. W. The incorporation of amino acids into protein in a cell-free system from yeast. Biochemistry. 1963 Jan-Feb;2:132–136. doi: 10.1021/bi00901a023. [DOI] [PubMed] [Google Scholar]
- SYLVEN B., TOBIAS C. A., MALMGREN H., OTTOSON R., THORELL B. Cyclic variations in the peptidase and catheptic activities of yeast cultures synchronized with respect to cell multiplication. Exp Cell Res. 1959 Jan;16(1):75–87. doi: 10.1016/0014-4827(59)90197-1. [DOI] [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T. Initiation of mammalian protein synthesis: the importance of ribosome and initiation factor quality for the efficiency of in vitro systems. J Mol Biol. 1973 Feb 19;73(3):329–349. doi: 10.1016/0022-2836(73)90346-x. [DOI] [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T. Translation of duck-globin messenger RNA in a partially purified mammalian cell-free system. Eur J Biochem. 1973 Apr;34(2):213–218. doi: 10.1111/j.1432-1033.1973.tb02748.x. [DOI] [PubMed] [Google Scholar]
- Shafritz D. A., Prichard P. M., Gilbert J. M., Anderson W. F. Separation of two factors, M1 and M2, required for poly U dependent polypeptide synthesis by rabbit reticulocyte ribosomes at low magnesium ion concentration. Biochem Biophys Res Commun. 1970 Feb 20;38(4):721–727. doi: 10.1016/0006-291x(70)90641-8. [DOI] [PubMed] [Google Scholar]
- Stavnezer J., Huang R. C. Synthesis of a mouse immunoglobulin light chain in a rabbit reticulocyte cell-free system. Nat New Biol. 1971 Apr 7;230(14):172–176. doi: 10.1038/newbio230172a0. [DOI] [PubMed] [Google Scholar]
- Stewart A. G., Gander E. S., Morel C., Luppis B., Scherrer K. Differential translation of duck- and rabbit-globin messenger RNAs in reticulocyte-lysate systems. Eur J Biochem. 1973 Apr;34(2):205–212. doi: 10.1111/j.1432-1033.1973.tb21105.x. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Udem S. A., Warner J. R. Ribosomal RNA synthesis in Saccharomyces cerevisiae. J Mol Biol. 1972 Mar 28;65(2):227–242. doi: 10.1016/0022-2836(72)90279-3. [DOI] [PubMed] [Google Scholar]
- Wigle D. T., Smith A. E. Specificity in initiation of protein synthesis in a fractionated mammalian cell-free system. Nat New Biol. 1973 Apr 4;242(118):136–140. doi: 10.1038/newbio242136a0. [DOI] [PubMed] [Google Scholar]