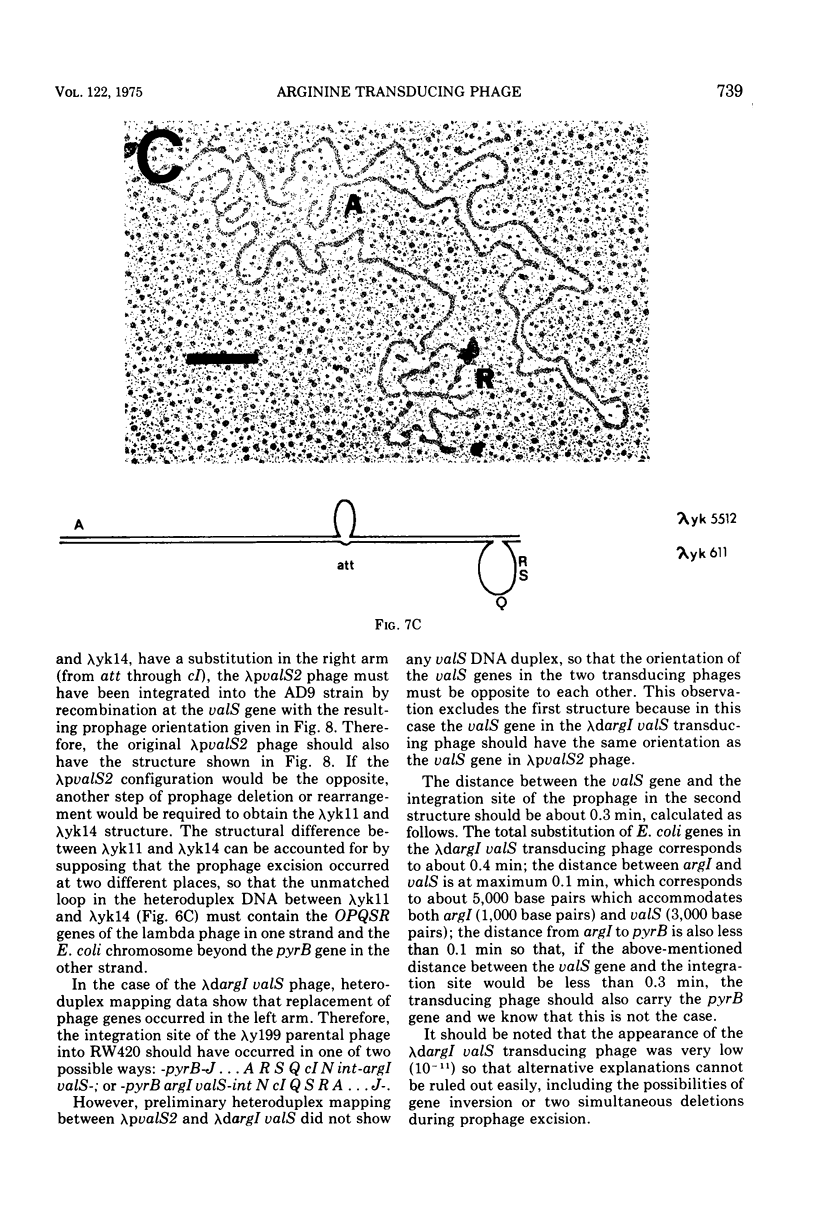
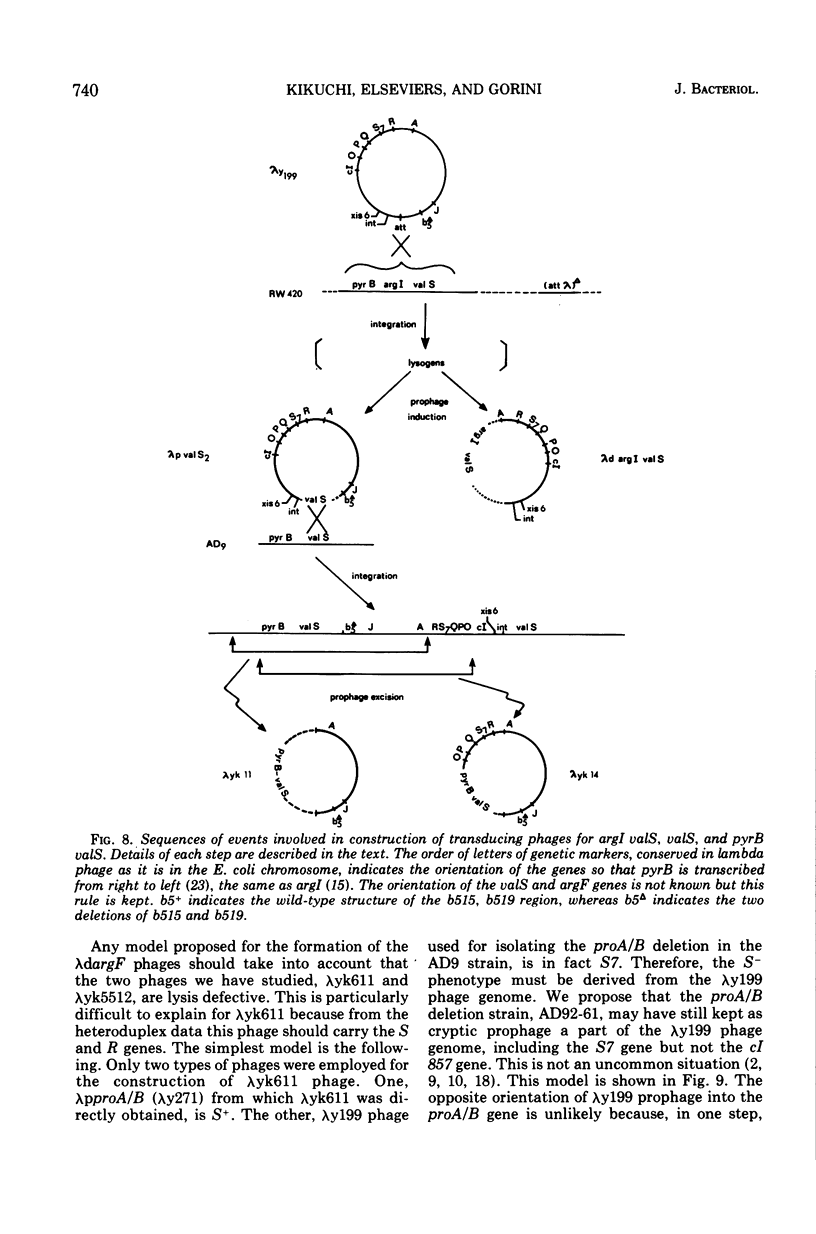
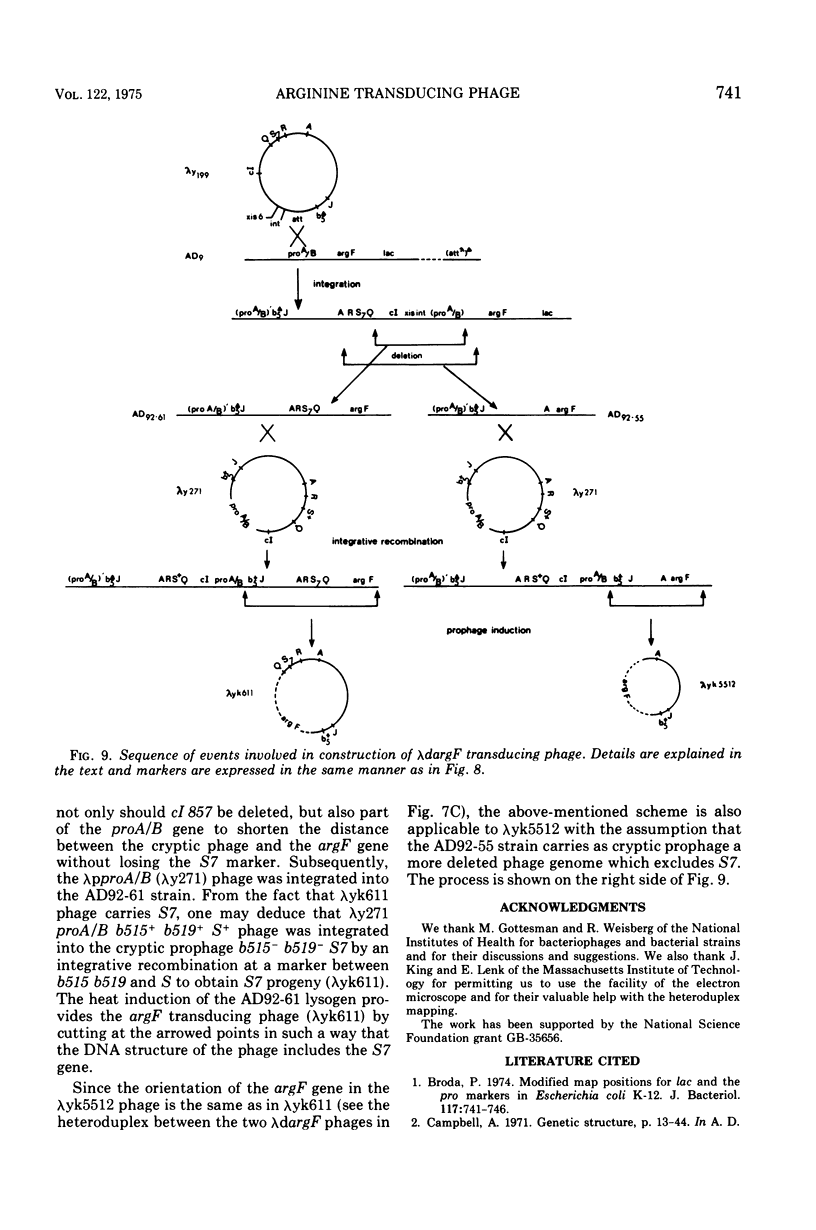
Abstract
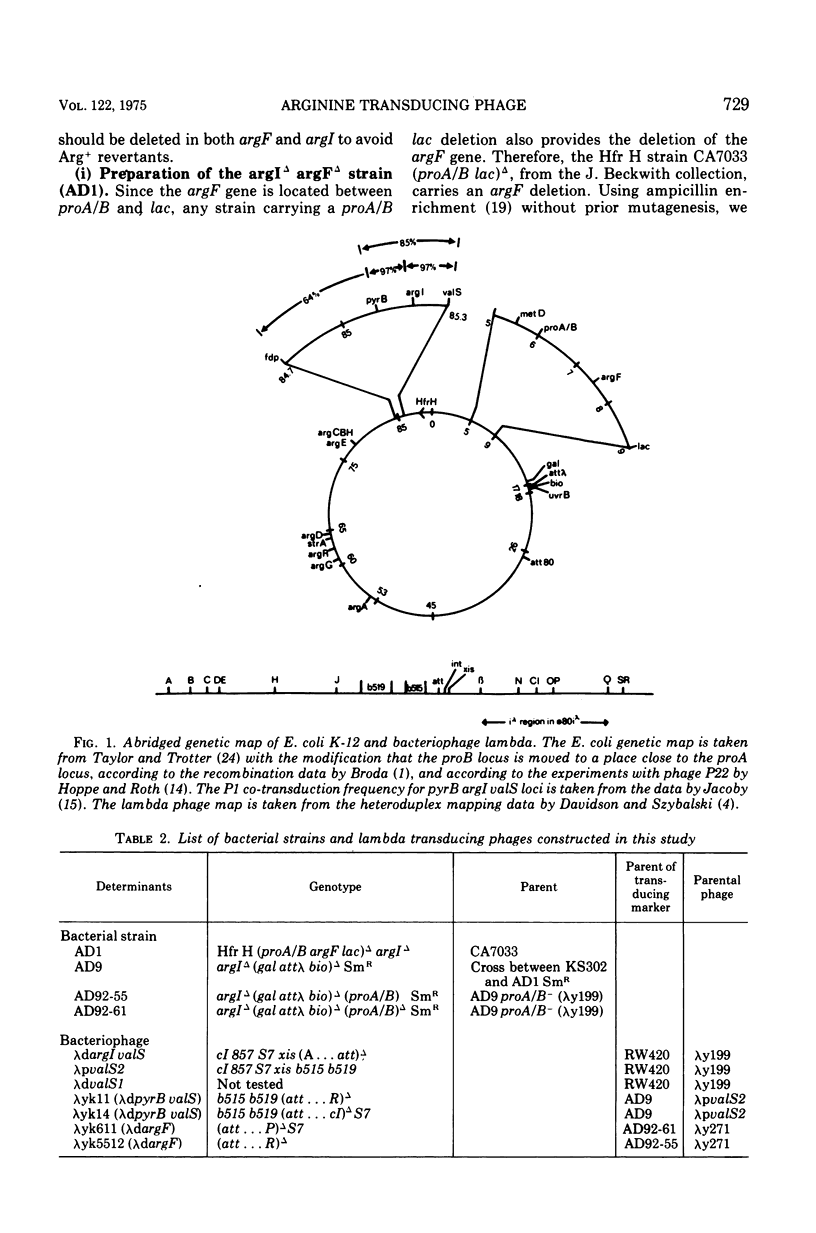
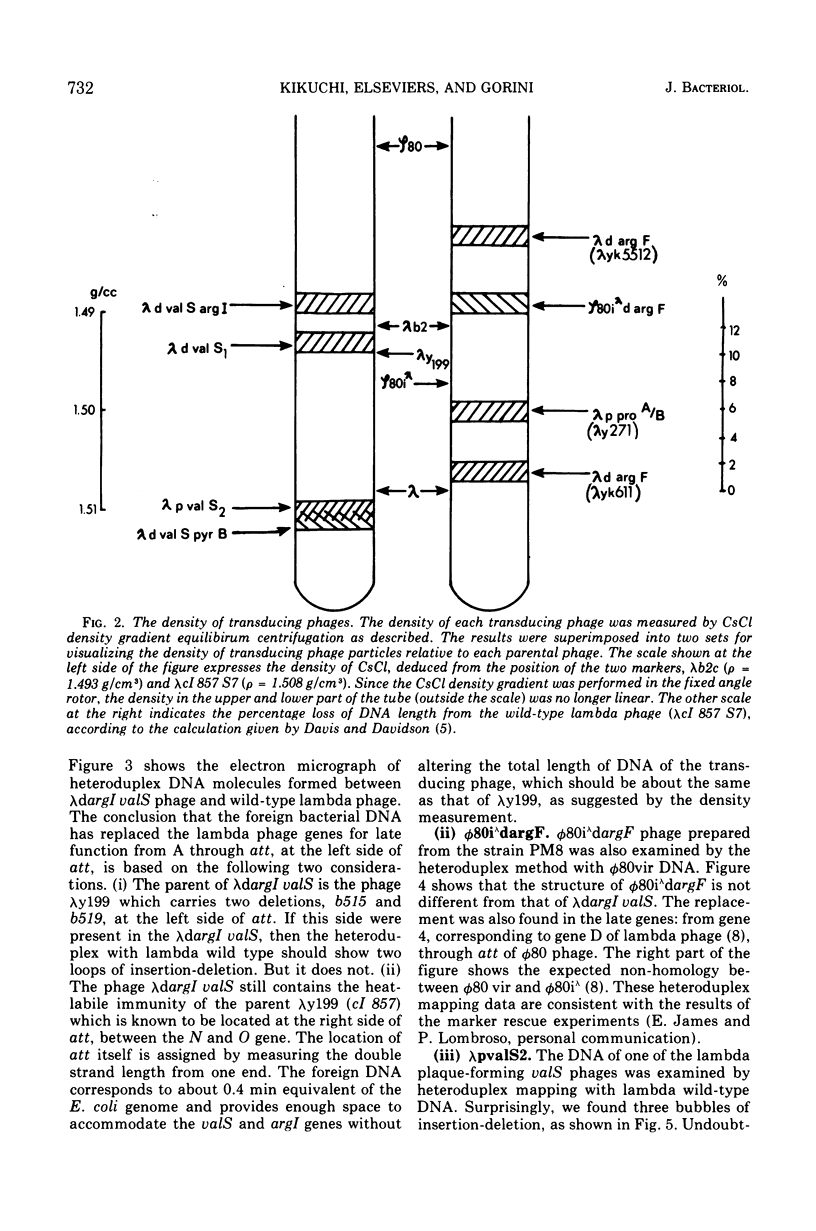
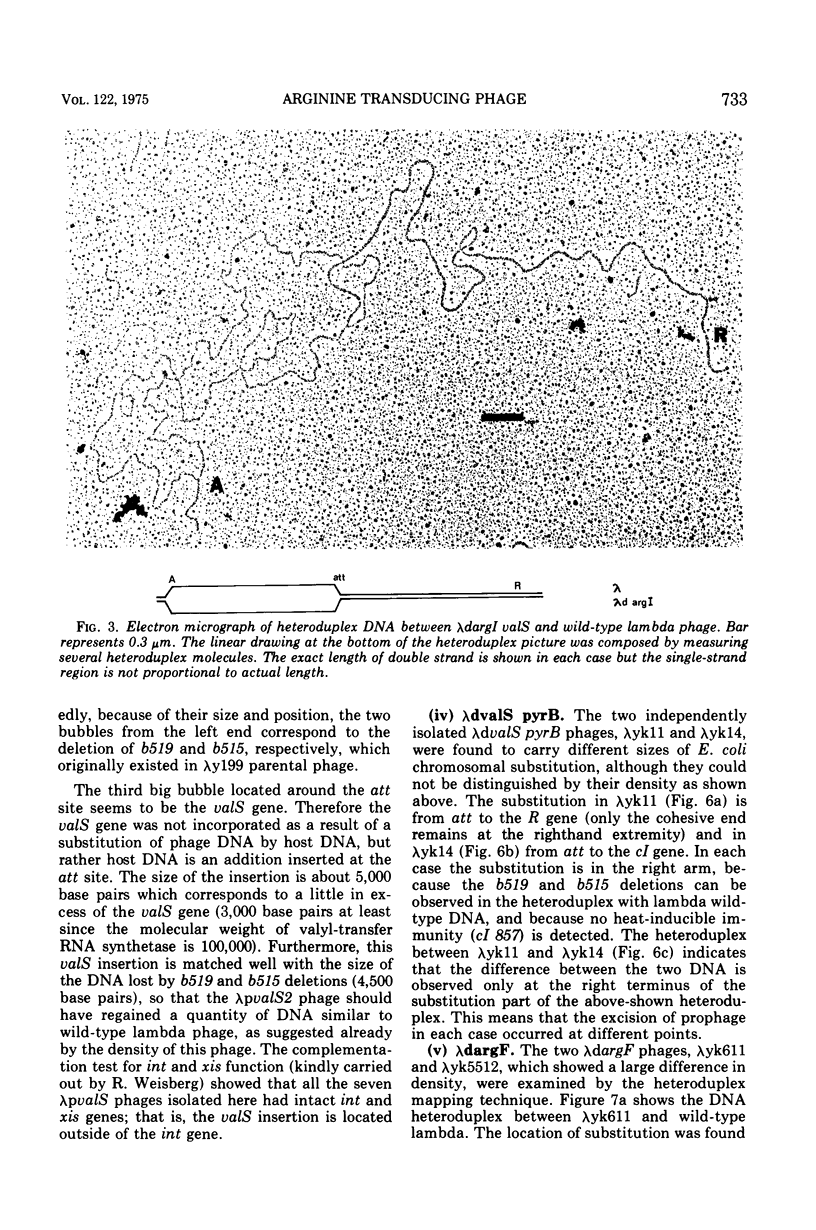
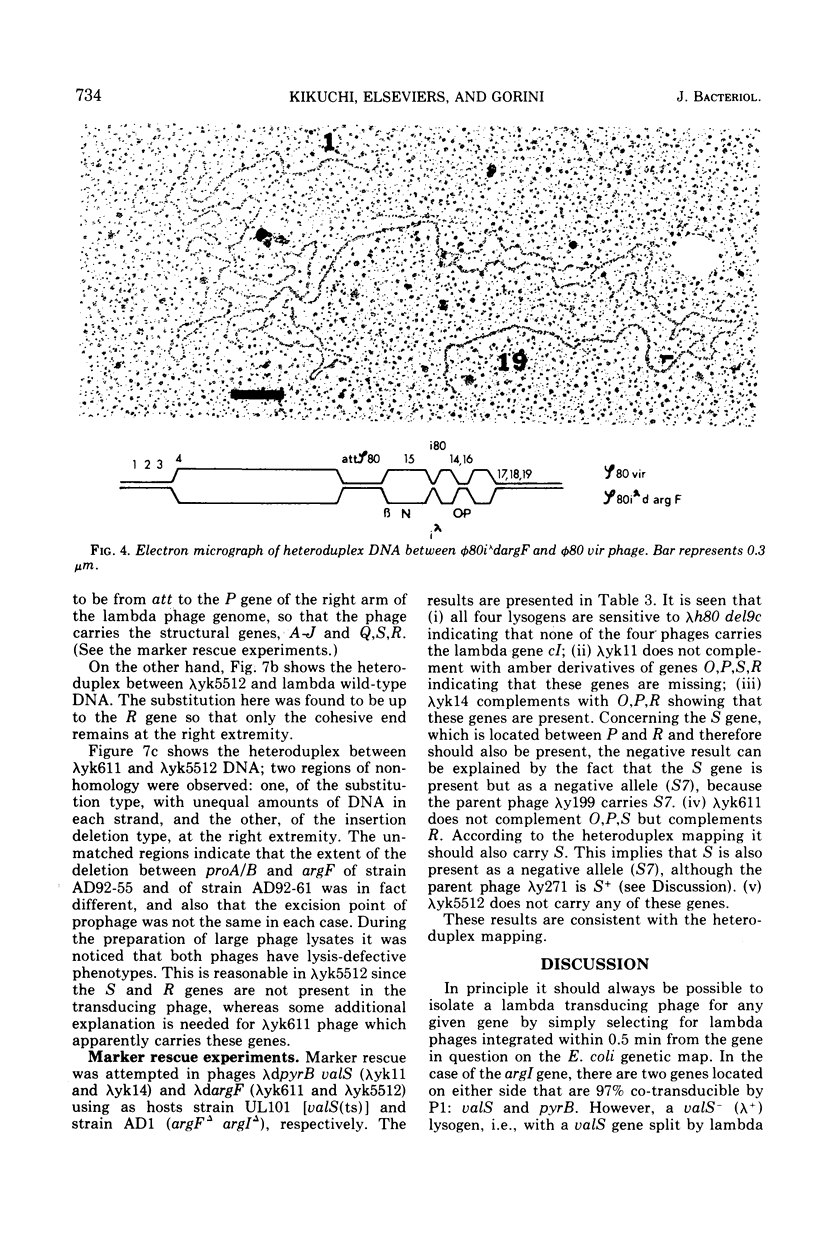
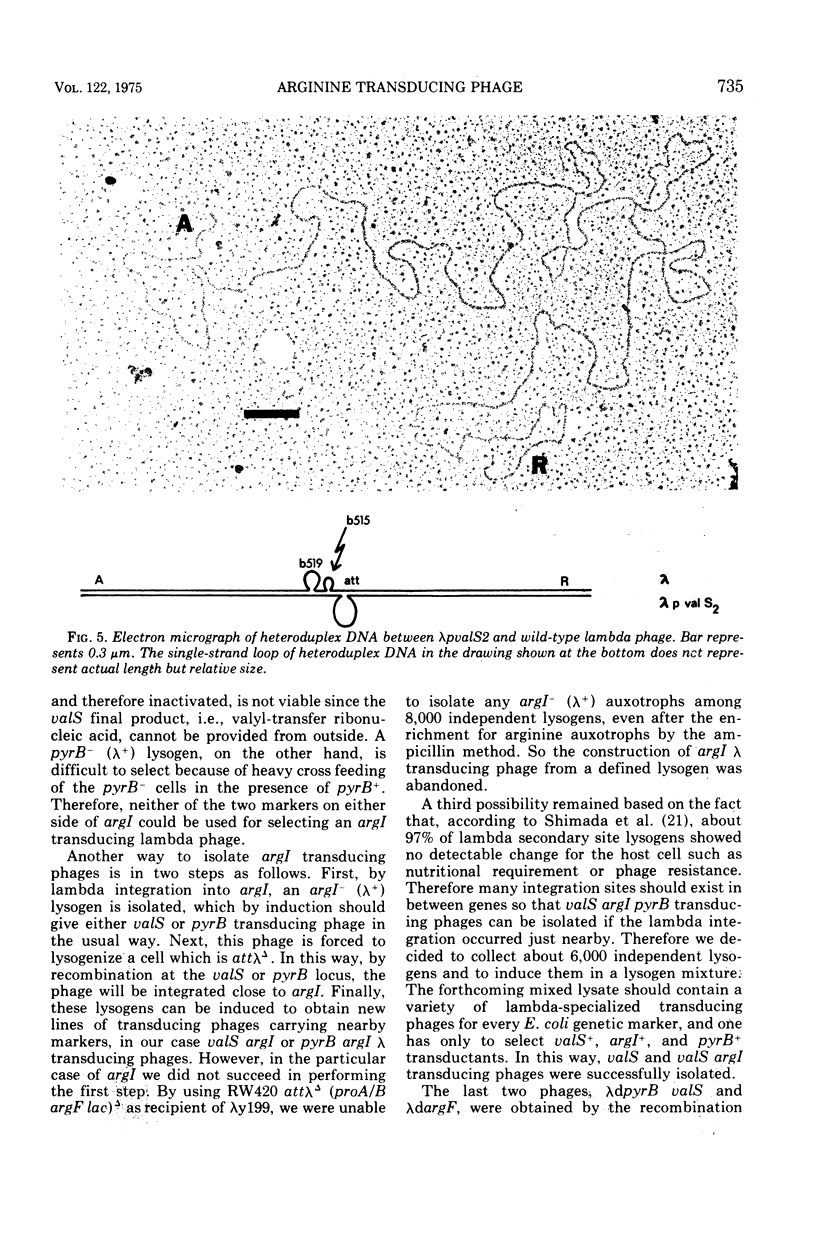
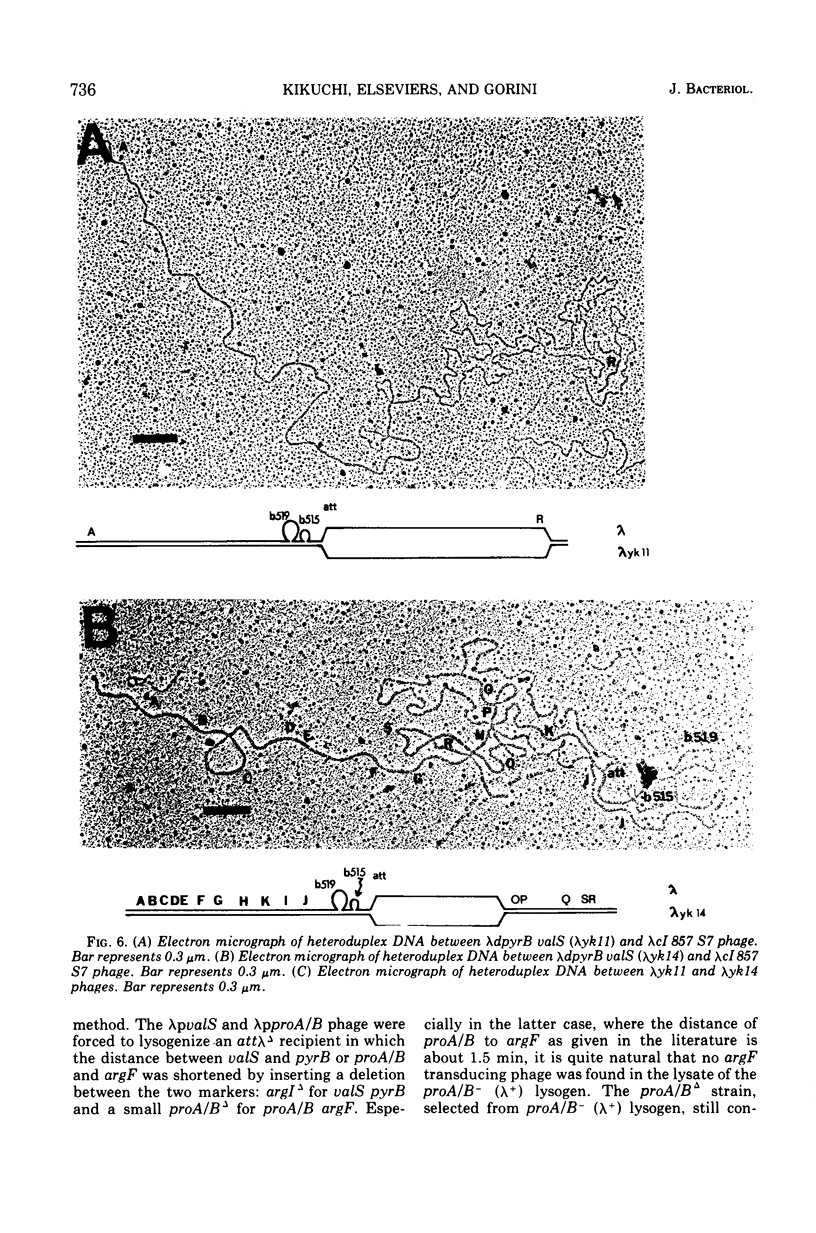
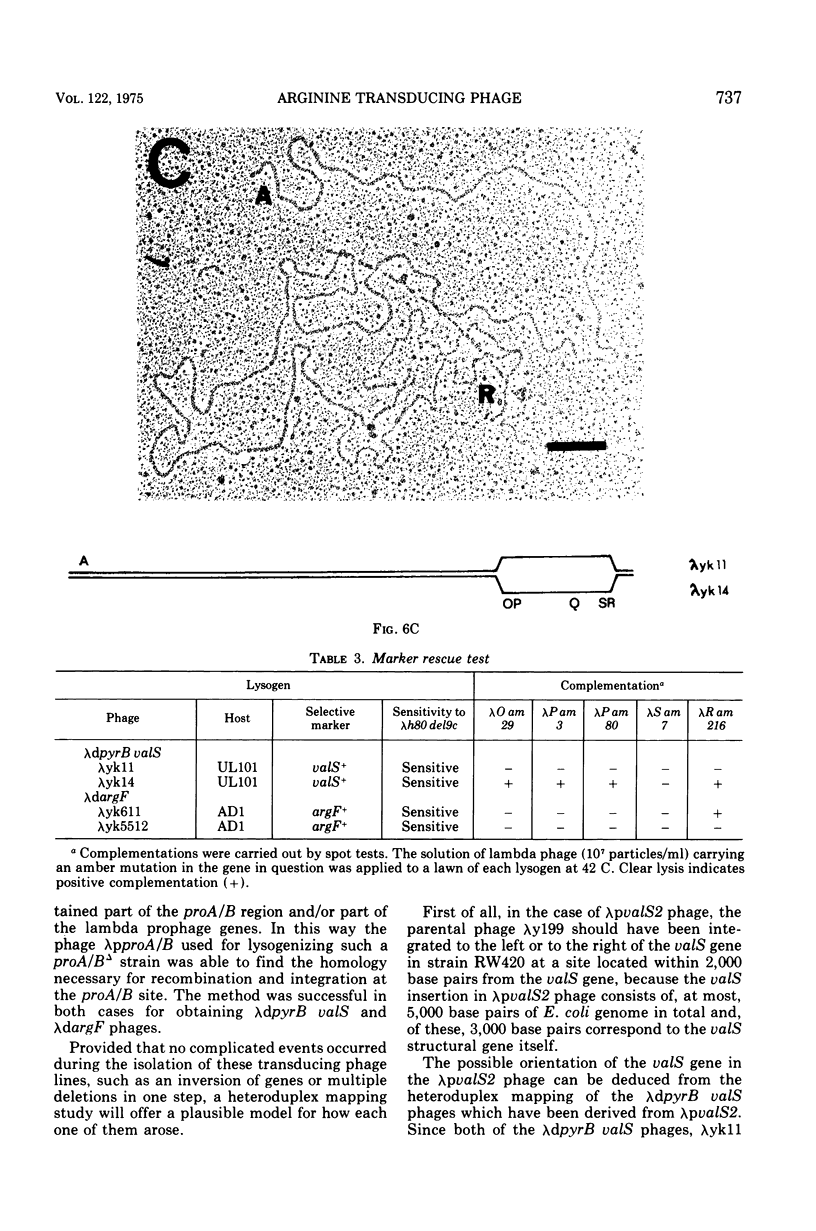
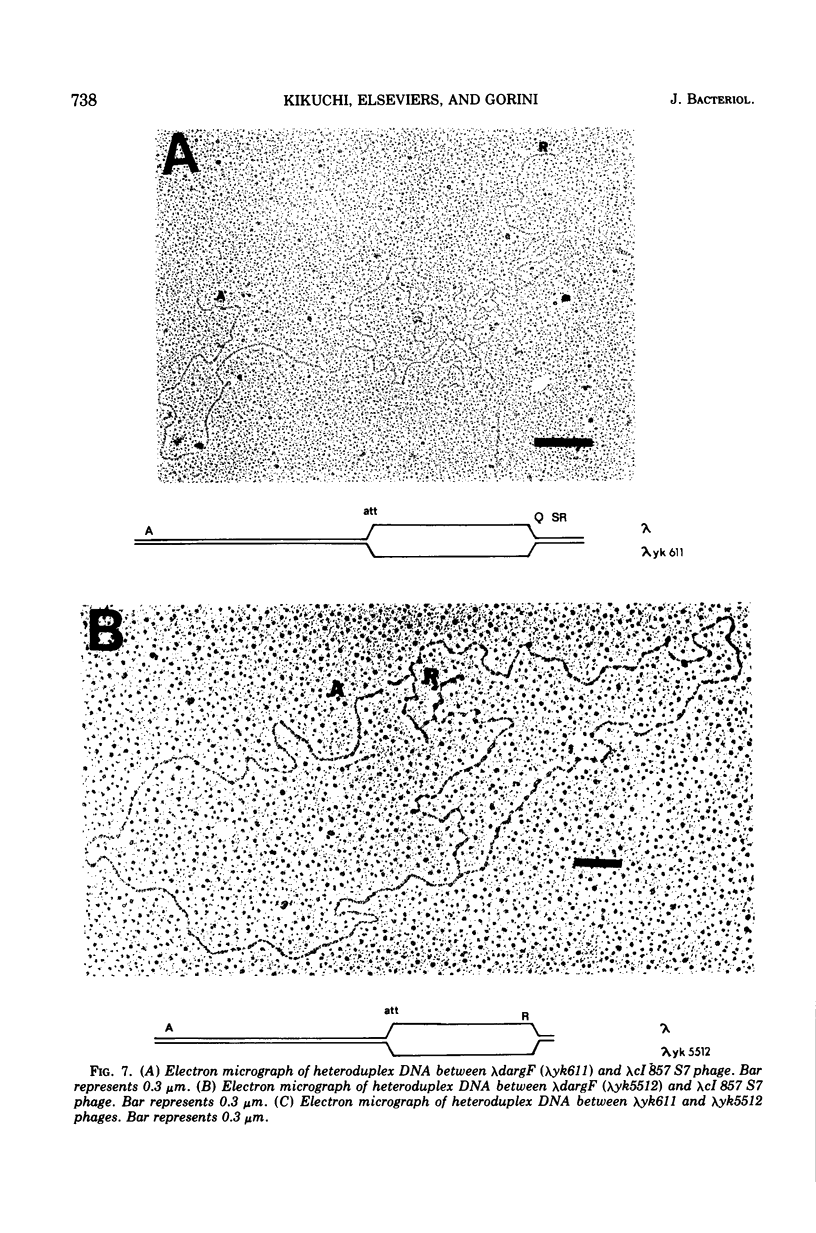
Two genes for ornithinetranscarbamylase exist in strain Escherichia coli K-12, argI, at 85 min, and argF, at 7 min. In an attempt to compare the deoxyribonucleic acid material of these two genes, the lambda transducing phages carrying a portion of the argI region, lambda dvalS argI, lambda pvalS, and lambda dvalS pyrB, and of the argF region, lambda dargF, have been isolated. Their structure, including that of phi 80dargF previously isolated, was studied by the method of heteroduplex mapping. In this paper, the results of this mapping are reported.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broda P. Modified map positions for lac and the pro markers in Escherichia coli K-12. J Bacteriol. 1974 Feb;117(2):741–746. doi: 10.1128/jb.117.2.741-746.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. W., Davidson N. Electron-microscopic visualization of deletion mutations. Proc Natl Acad Sci U S A. 1968 May;60(1):243–250. doi: 10.1073/pnas.60.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. W., Parkinson J. S. Deletion mutants of bacteriophage lambda. 3. Physical structure of att-phi. J Mol Biol. 1971 Mar 14;56(2):403–423. doi: 10.1016/0022-2836(71)90473-6. [DOI] [PubMed] [Google Scholar]
- FISCHER-FANTUZZI L., CALEF E. A TYPE OF LAMBDA PROPHAGE UNABLE TO CONFER IMMUNITY. Virology. 1964 Jun;23:209–216. doi: 10.1016/0042-6822(64)90284-3. [DOI] [PubMed] [Google Scholar]
- Fischer-Fantuzzi L. Integration of lambda and lambda-b2 genomes in nonimmune host bacteria carrying a lambda cryptic prophage. Virology. 1967 May;32(1):18–32. doi: 10.1016/0042-6822(67)90248-6. [DOI] [PubMed] [Google Scholar]
- Glansdorff N., Sand G., Verhoef C. The dual genetic control of ornithine transcarbamylase synthesis in Escherichia coli K12. Mutat Res. 1967 Nov-Dec;4(6):743–751. doi: 10.1016/0027-5107(67)90083-8. [DOI] [PubMed] [Google Scholar]
- Gottesman M. E., Yarmolinsky M. B. Integration-negative mutants of bacteriophage lambda. J Mol Biol. 1968 Feb 14;31(3):487–505. doi: 10.1016/0022-2836(68)90423-3. [DOI] [PubMed] [Google Scholar]
- Gottesman S., Beckwith J. R. Directed transposition of the arabinose operon: a technique for the isolation of specialized transducing bacteriophages for any Escherichia coli gene. J Mol Biol. 1969 Aug 28;44(1):117–127. doi: 10.1016/0022-2836(69)90408-2. [DOI] [PubMed] [Google Scholar]
- Hoppe I., Roth J. Specialized transducing phages derived from salmonella phage P22. Genetics. 1974 Apr;76(4):633–654. doi: 10.1093/genetics/76.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby G. A. Mapping the gene determining ornithine transcarbamylase and its operator in Escherichia coli B. J Bacteriol. 1971 Nov;108(2):645–651. doi: 10.1128/jb.108.2.645-651.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye R., Barravecchio J., Roth J. Isolation of P22 specialized transducing phage followong F'-episome fusion. Genetics. 1974 Apr;76(4):655–667. doi: 10.1093/genetics/76.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrain C., Halleux P., Stalon V., Glansdorff N. The dual genetic control of ornithine carbamolytransferase in Escherichia coli. A case of bacterial hybrid enzymes. Eur J Biochem. 1972 May;27(1):93–102. doi: 10.1111/j.1432-1033.1972.tb01814.x. [DOI] [PubMed] [Google Scholar]
- Marchelli C., Pica L., Soller A. The cryptogenic factor in lambda. Virology. 1968 Apr;34(4):650–663. doi: 10.1016/0042-6822(68)90086-x. [DOI] [PubMed] [Google Scholar]
- Shimada K., Weisberg R. A., Gottesman M. E. Prophage lambda at unusual chromosomal locations. I. Location of the secondary attachment sites and the properties of the lysogens. J Mol Biol. 1972 Feb 14;63(3):483–503. doi: 10.1016/0022-2836(72)90443-3. [DOI] [PubMed] [Google Scholar]
- Shimada K., Weisberg R. A., Gottesman M. E. Prophage lambda at unusual chromosomal locations. II. Mutations induced by bacteriophage lambda in Escherichia coli K12. J Mol Biol. 1973 Oct 25;80(2):297–314. doi: 10.1016/0022-2836(73)90174-5. [DOI] [PubMed] [Google Scholar]
- Shimada K., Weisberg R., Gottesman M. E. coli mutants produced by the insertion of bacteriophage lambda DNA. Genetics. 1973 Apr;73(Suppl):81–83. [PubMed] [Google Scholar]
- Syvanen J. M., Roth J. R. Structural genes for catalytic and regulatory subunits of aspartate transcarbamylase. J Mol Biol. 1973 May 25;76(3):363–378. doi: 10.1016/0022-2836(73)90510-x. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye B. K., Chan R. K., Botstein D. Packaging of an oversize transducing genome by Salmonella phage P22. J Mol Biol. 1974 Jan 5;85(4):485–500. doi: 10.1016/0022-2836(74)90311-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]









