Abstract
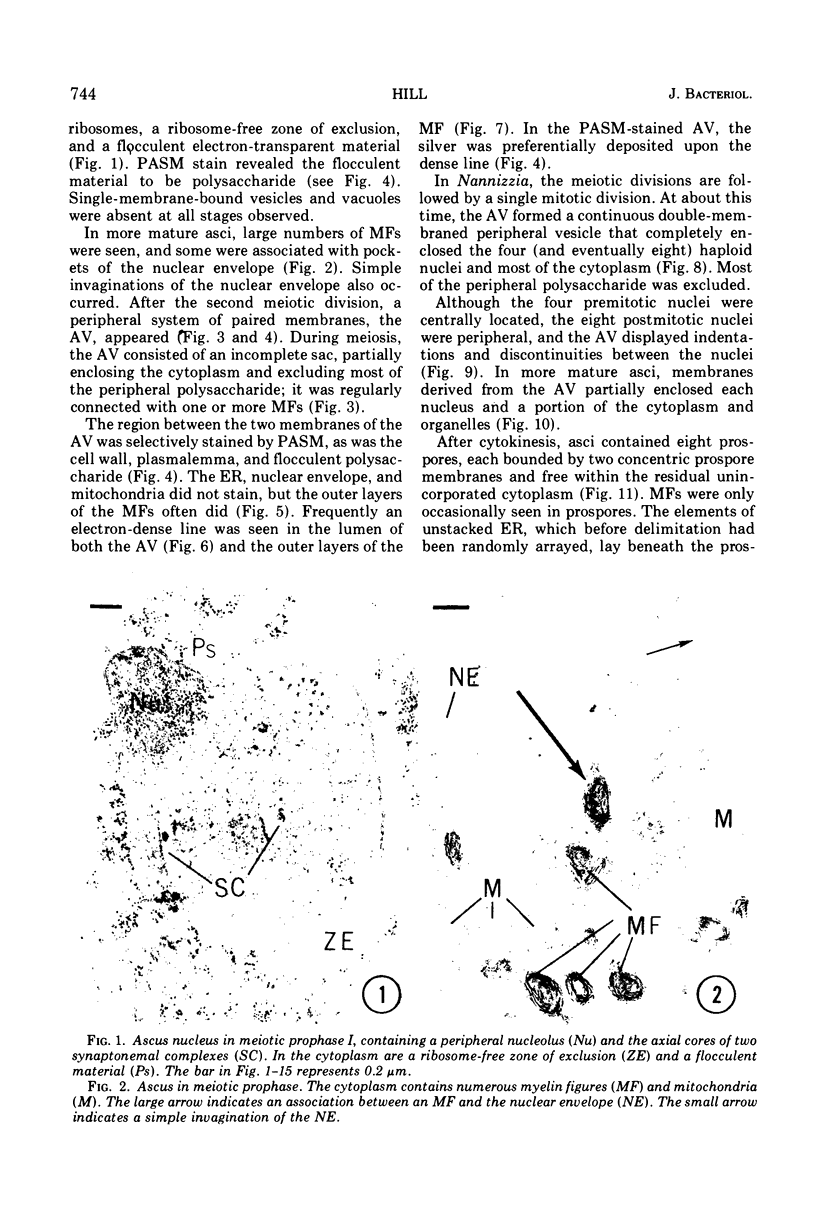
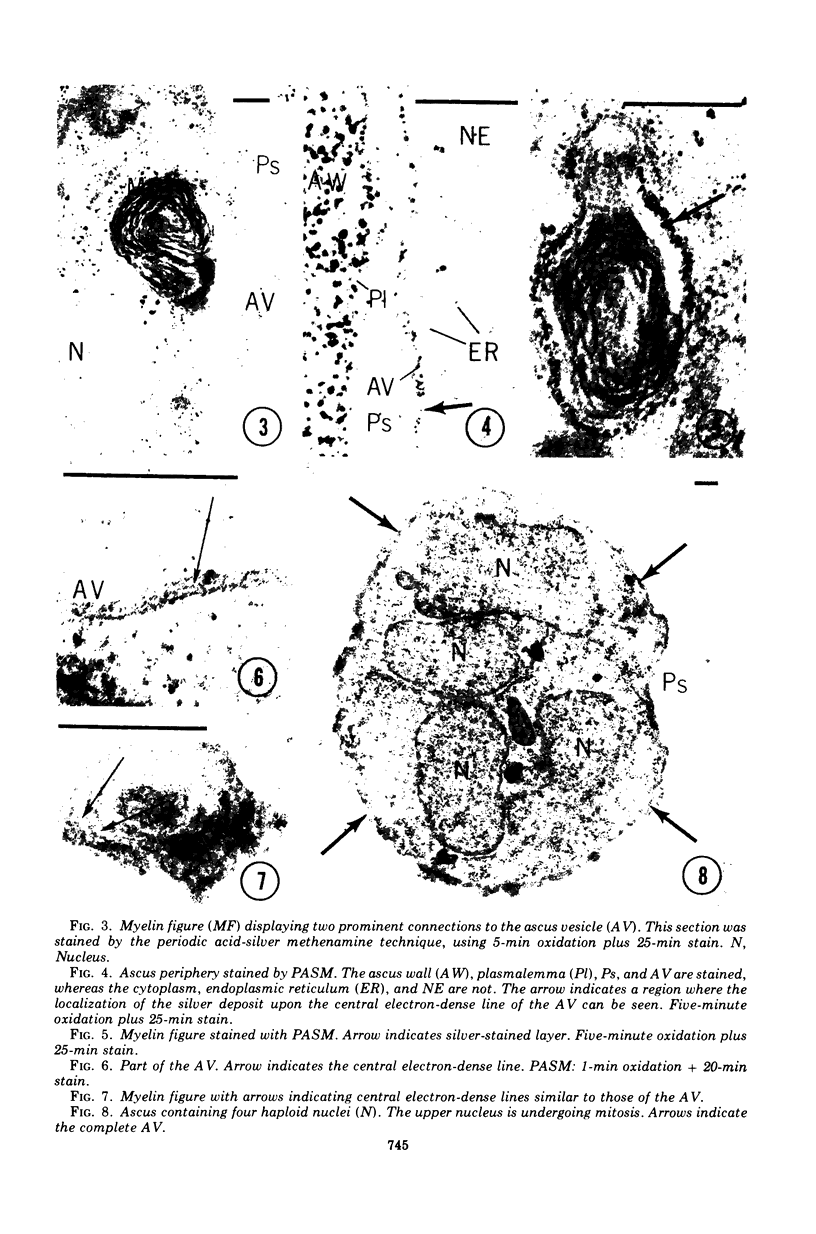
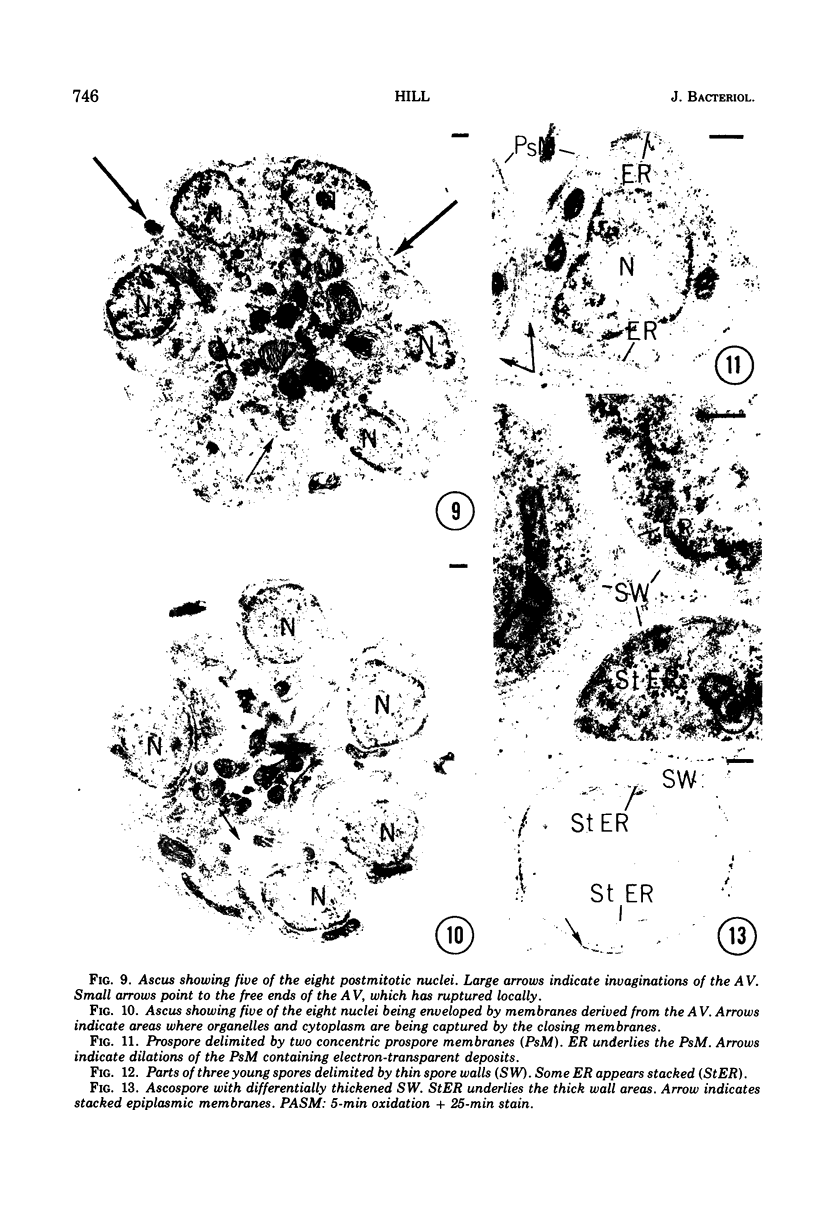
Ascosporogenesis in Nannizzia gypsea was studied by electron microscopy. Development of ascospores began with the formation of an ascus vesicle composed of two paired unit membranes. Myelin figures consisting of coiled or concentric membranes were regularly connected with the growing ascus vesicle. Both the ascus vesicle and the myelin figures possessed an electron-dense line between paired membranes, and both were stained by the periodic acid-silver methenamine technique. Invagination of the ascus vesicle about the haploid nuclei resulted in eight uninucleate prospores bounded by two concentric membranes. Spore wall material was deposited between the two membranes of the prospores, and deposition was greatest in areas of the wall overlying stacked elements of endoplasmic reticulum. A single myelin figure surrounded by a polysaccharide halo was observed in the spore.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black S. H., Gorman C. The cytology of Hansenula. 3. Nuclear segregation and envelopment during ascosporogenesis in Hansenula wingei. Arch Mikrobiol. 1971;79(3):231–248. [PubMed] [Google Scholar]
- Carroll G. C. The ultrastructure of ascospore delimitation in Saccobolus kerverni. J Cell Biol. 1967 Apr;33(1):218–224. doi: 10.1083/jcb.33.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole G. T., Aldrich H. C. Demonstration of myelin figures in unfixed, freeze-etched fungus spores. J Cell Biol. 1971 Dec;51(3):873–874. doi: 10.1083/jcb.51.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martino C., Zamboni L. Silver methenamine stain for electron microscopy. J Ultrastruct Res. 1967 Aug;19(3):273–282. doi: 10.1016/s0022-5320(67)80221-1. [DOI] [PubMed] [Google Scholar]
- Gil F. Mesosomes: their role in the delimitation of the ascospore. Mycopathol Mycol Appl. 1973 Apr 30;49(4):243–247. doi: 10.1007/BF02050717. [DOI] [PubMed] [Google Scholar]
- Lynn R. R., Magee P. T. Development of the spore wall during ascospore formation in Saccharomyces cerevisiae. J Cell Biol. 1970 Mar;44(3):688–692. doi: 10.1083/jcb.44.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre J. A., Gilula N. B., Karnovsky M. J. Cryoprotectant-induced redistribution of intramembranous particles in mouse lymphocytes. J Cell Biol. 1974 Jan;60(1):192–203. doi: 10.1083/jcb.60.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens P. B. Fine structure of ascospore development in the yeast Saccharomyces cerevisiae. Can J Microbiol. 1971 Apr;17(4):507–510. doi: 10.1139/m71-084. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves F., Jr The fine structure of ascospore formation in Pyronema domesticum. Mycologia. 1967 Nov-Dec;59(6):1018–1033. [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- VANBREUSEGHEM R. Technique biologique pour l'isolement des dermatophytes du sol. Ann Soc Belg Med Trop (1920) 1952 Apr 30;32(2):173–178. [PubMed] [Google Scholar]
- Weitzman I., Silva-Hutner M. Non-keratinous agar media as substrates for the ascigerous state in certain members of the Gymnoascaceae pathogenic for man and animals. Sabouraudia. 1967 Jun;5(4):335–340. doi: 10.1080/00362176785190611. [DOI] [PubMed] [Google Scholar]