Abstract
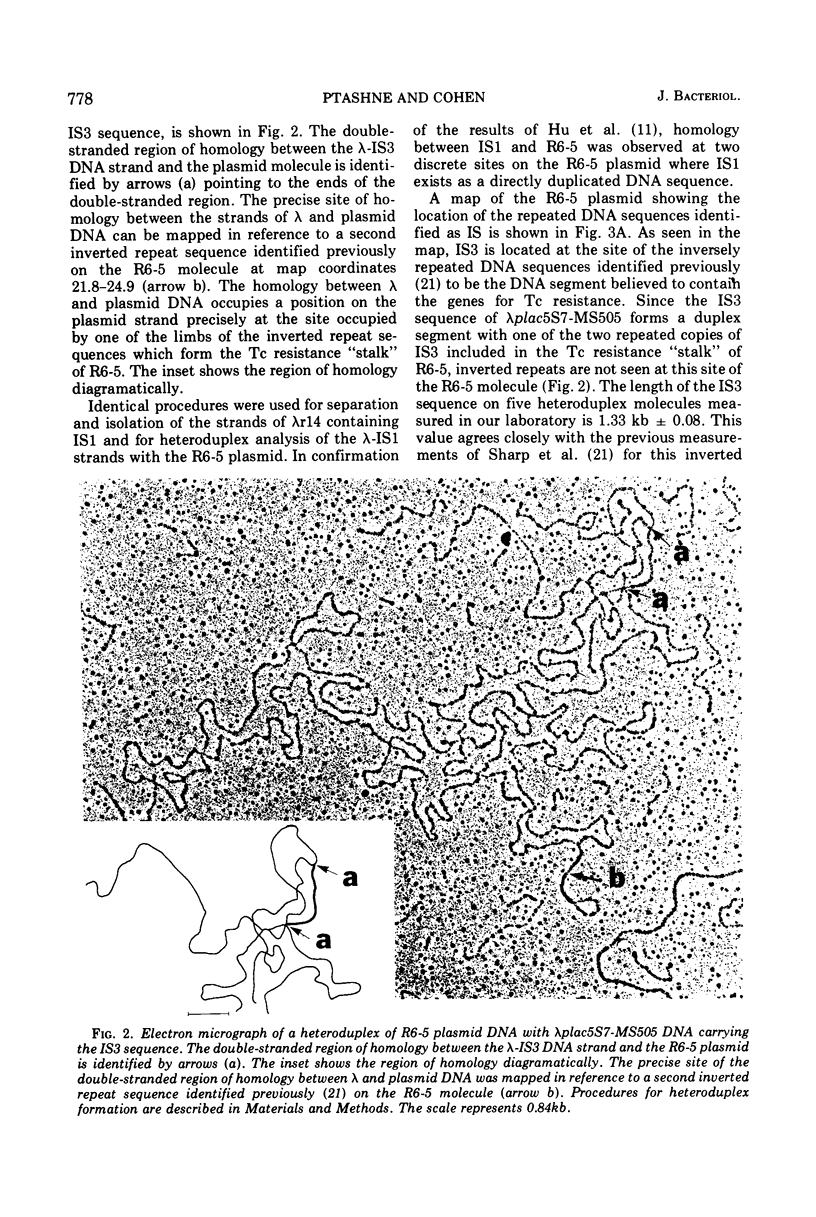
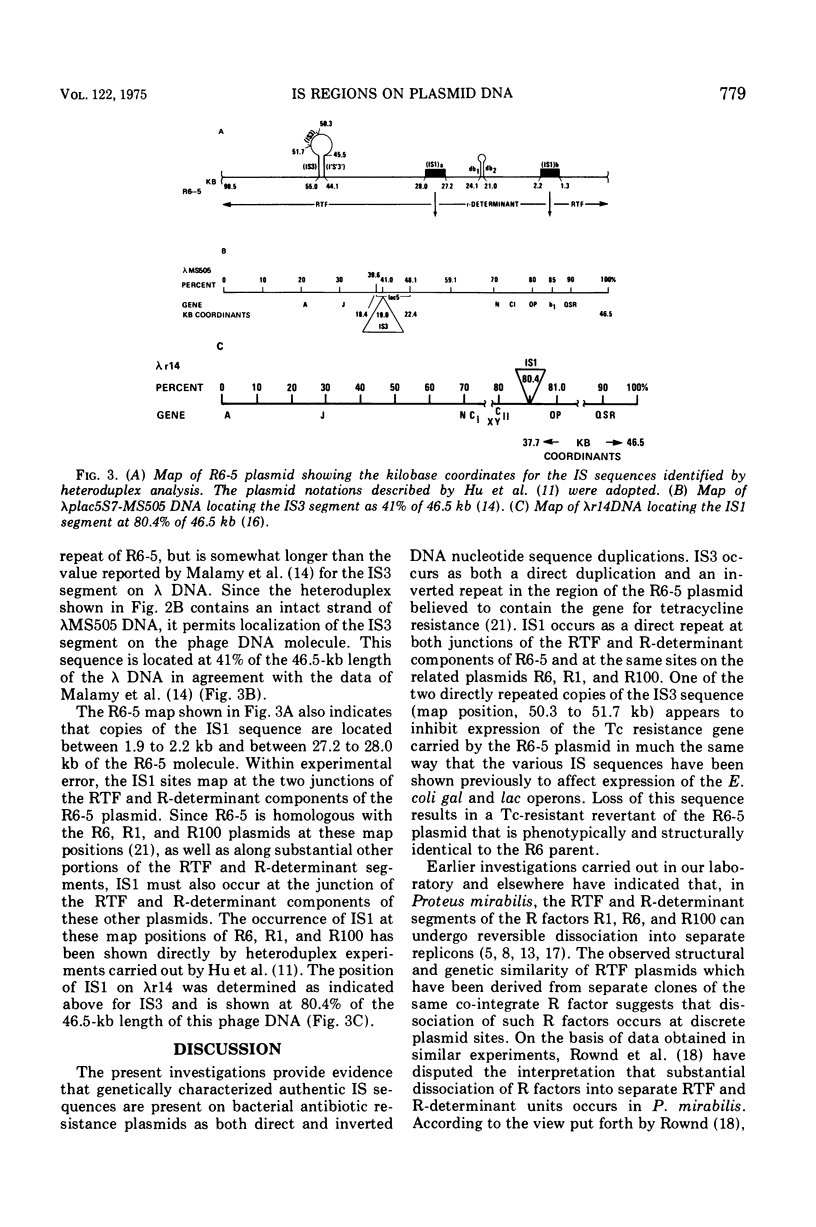
Insertion sequence (IS) regions have been identified previously as a cause of strongly polar mutations in Escherichia coli and several bacteriophages. The present experiments indicate that genetically characterized IS regions occur on bacterial plasmid deoxyribonucleic acid (DNA) as both direct and inverted DNA sequence duplications. The DNA insertion which has been shown previously (Sharp et al., 1973) to control expression of tetracycline resistance in the R6-5 plasmid, and which occurs as directly and inversely repeated DNA sequences adjacent to the region believed to contain the tetracycline resistance gene, has been identified as IS3. A second genetically characterized insertion sequence (IS1) has been identified as a direct DNA duplication occurring at both junctions of the resistance transfer factor and R-determinant components of R6-5 and related plasmids. A model is presented for the reversible dissociation of resistance transfer factor and R-determinant components of co-integrate R plasmids at the sites of DNA sequence homology provided by the repeated IS regions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelberg E. A., Bergquist P. The stabilization of episomal integration by genetic inversion: a general hypothesis. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2061–2065. doi: 10.1073/pnas.69.8.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C. Genetic expression in bacteriophage lambda. 3. Inhibition of Escherichia coli nucleic acid and protein synthesis during lambda development. J Mol Biol. 1970 May 14;49(3):557–575. doi: 10.1016/0022-2836(70)90281-0. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C. Recircularization and autonomous replication of a sheared R-factor DNA segment in Escherichia coli transformants. Proc Natl Acad Sci U S A. 1973 May;70(5):1293–1297. doi: 10.1073/pnas.70.5.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Hurwitz J. Transcription of complementary strands of phage lambda-DNA in vivo and in vitro. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1759–1766. doi: 10.1073/pnas.57.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Miller C. A. Non-chromosomal antibiotic resistance in bacteria. 3. Isolation of the discrete transfer unit of the R-factor R1. Proc Natl Acad Sci U S A. 1970 Oct;67(2):510–516. doi: 10.1073/pnas.67.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Miller C. A. Non-chromosomal antibiotic resistance in bacteria. II. Molecular nature of R-factors isolated from Proteus mirabilis and Escherichia coli. J Mol Biol. 1970 Jun 28;50(3):671–687. doi: 10.1016/0022-2836(70)90092-6. [DOI] [PubMed] [Google Scholar]
- Gilbert W., Maxam A. The nucleotide sequence of the lac operator. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3581–3584. doi: 10.1073/pnas.70.12.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapala D. K., Falkow S. Physical studies of the drug-resistance transfer factor in Proteus. J Bacteriol. 1971 Apr;106(1):294–295. doi: 10.1128/jb.106.1.294-295.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch H. J., Starlinger P., Brachet P. Two kinds of insertions in bacterial genes. Mol Gen Genet. 1972;119(3):191–206. doi: 10.1007/BF00333858. [DOI] [PubMed] [Google Scholar]
- Hsu M. T., Davidson N. Electron microscope heteroduplex study of the heterogeneity of Mu phage and prophage DNA. Virology. 1974 Mar;58(1):229–239. doi: 10.1016/0042-6822(74)90157-3. [DOI] [PubMed] [Google Scholar]
- Hu S., Otsubo E., Davidson N., Saedler H. Electron microscope heteroduplex studies of sequence relations among bacterial plasmids: identification and mapping of the insertion sequences IS1 and IS2 in F and R plasmids. J Bacteriol. 1975 May;122(2):764–775. doi: 10.1128/jb.122.2.764-775.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Smith H. O. A restriction enzyme from Hemophilus influenzae. II. J Mol Biol. 1970 Jul 28;51(2):393–409. doi: 10.1016/0022-2836(70)90150-6. [DOI] [PubMed] [Google Scholar]
- Kopecko D. J., Cohen S. N. Site specific recA--independent recombination between bacterial plasmids: involvement of palindromes at the recombinational loci. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1373–1377. doi: 10.1073/pnas.72.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecko D. J., Punch J. D. The problems of drug-resistant pathogenic bacteria. Regulation of R-factor replication in Proteus mirabilis. Ann N Y Acad Sci. 1971 Jun 11;182:201–216. doi: 10.1111/j.1749-6632.1971.tb30657.x. [DOI] [PubMed] [Google Scholar]
- Malamy M. H., Fiandt M., Szybalski W. Electron microscopy of polar insertions in the lac operon of Escherichia coli. Mol Gen Genet. 1972;119(3):207–222. doi: 10.1007/BF00333859. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Ptashne M., Barrell B. G., Donelson J. Sequence of a repressor-binding site in the DNA of bacteriophage lamda. Nature. 1974 Aug 2;250(465):394–397. doi: 10.1038/250394a0. [DOI] [PubMed] [Google Scholar]
- Michaelis G., Saedler H., Venkov P., Starlinger P. Two insertions in the galactose operon having different sizes but homologous DNA sequences. Mol Gen Genet. 1969 Aug 15;104(4):371–377. doi: 10.1007/BF00334236. [DOI] [PubMed] [Google Scholar]
- Nisioka T., Mitani M., Clowes R. Composite circular forms of R factor deoxyribonucleic acid molecules. J Bacteriol. 1969 Jan;97(1):376–385. doi: 10.1128/jb.97.1.376-385.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saedler H., Heiss B. Multiple copies of the insertion-DNA sequences IS1 and IS2 in the chromosome of E. coli K-12. Mol Gen Genet. 1973 May 9;122(3):267–277. doi: 10.1007/BF00278602. [DOI] [PubMed] [Google Scholar]
- Saedler H., Reif H. J., Hu S., Davidson N. IS2, a genetic element for turn-off and turn-on of gene activity in E. coli. Mol Gen Genet. 1974;132(4):265–289. doi: 10.1007/BF00268569. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Cohen S. N., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. II. Structure of drug resistance (R) factors and F factors. J Mol Biol. 1973 Apr 5;75(2):235–255. doi: 10.1016/0022-2836(73)90018-1. [DOI] [PubMed] [Google Scholar]
- Silver R. P., Falkow S. Studies on resistance transfer factor deoxyribonucleic acid in Escherichia coli. J Bacteriol. 1970 Oct;104(1):340–344. doi: 10.1128/jb.104.1.340-344.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K., Hradecna Z., Szybalski W. Asymmetric distribution of the transcribing regions on the complementary strands of coliphage lambda DNA. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1618–1625. doi: 10.1073/pnas.57.6.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. A., Thomas C. A., Jr Palindromes in chromosomes. J Mol Biol. 1974 Mar 25;84(1):115–138. doi: 10.1016/0022-2836(74)90216-2. [DOI] [PubMed] [Google Scholar]
- van Embden J., Cohen S. N. Molecular and genetic studies of an R factor system consisting of independent transfer and drug resistance plasmids. J Bacteriol. 1973 Nov;116(2):699–709. doi: 10.1128/jb.116.2.699-709.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]




