Abstract
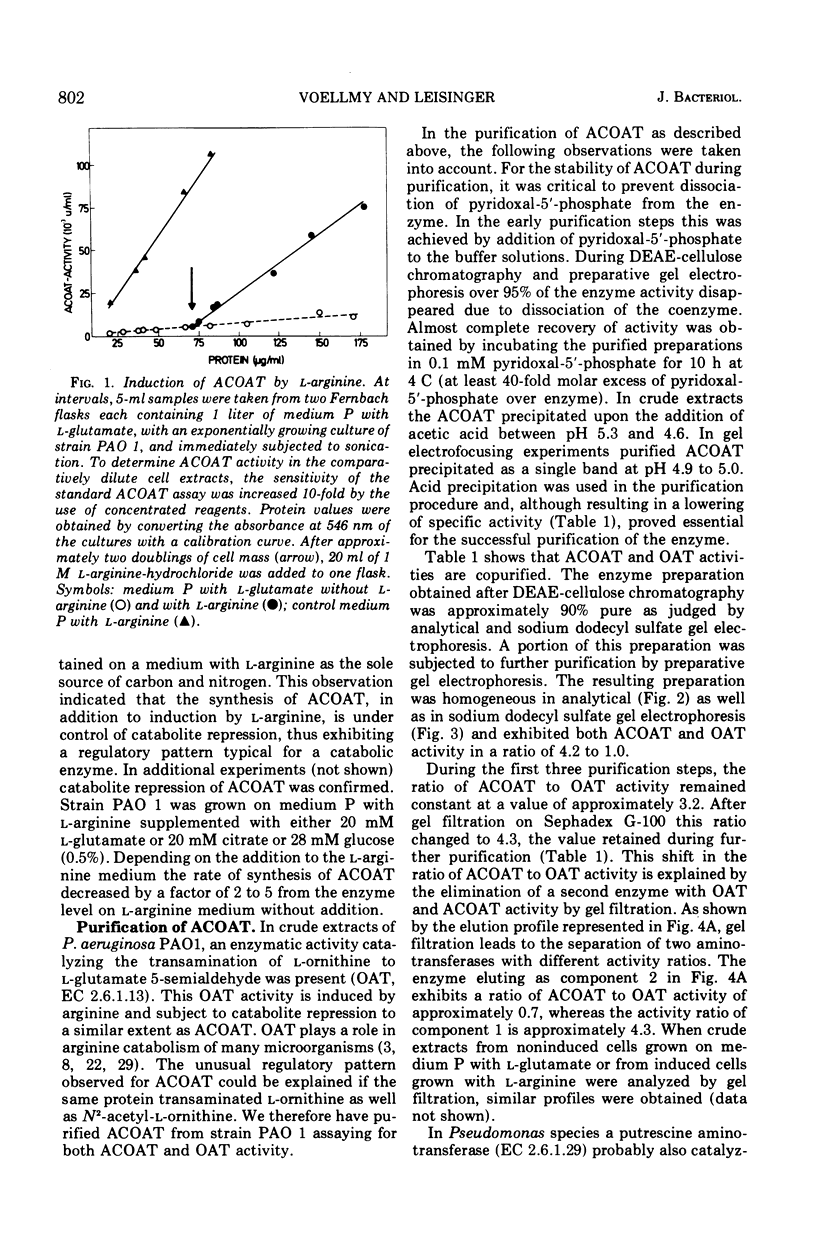
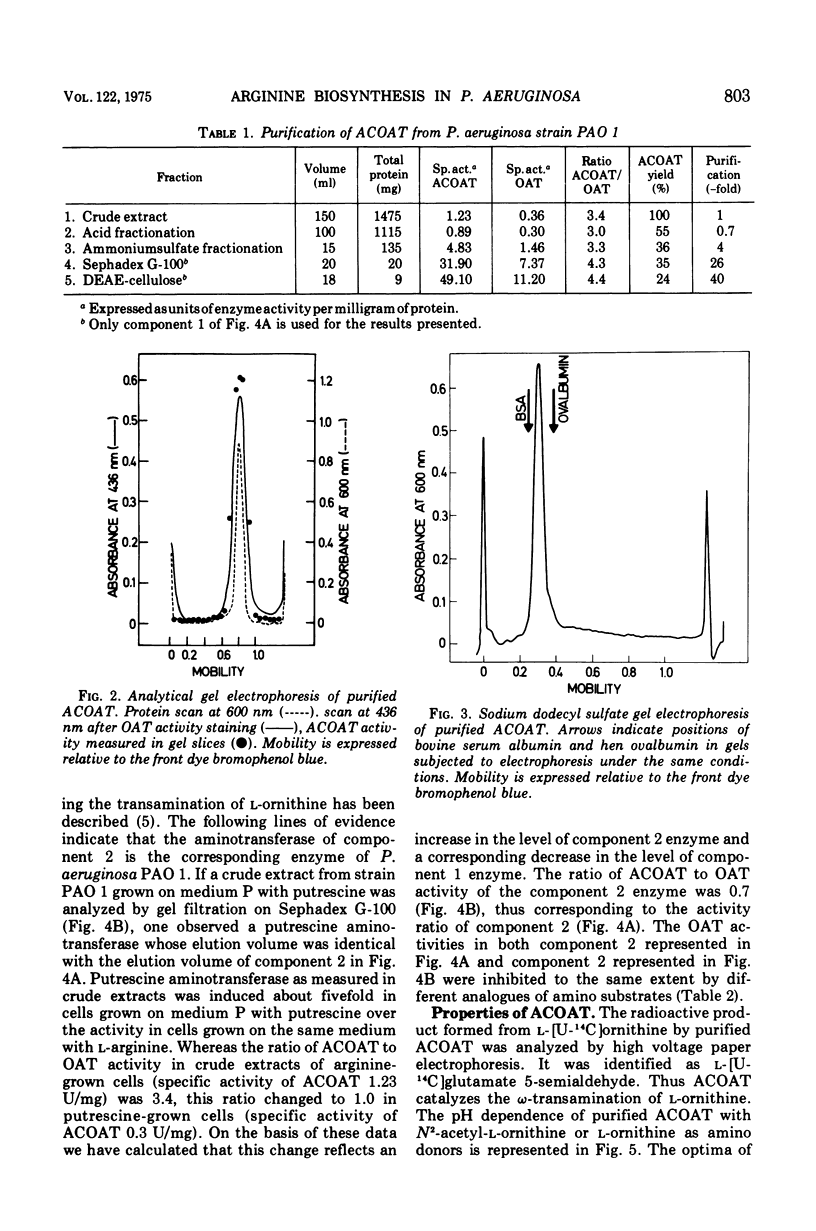
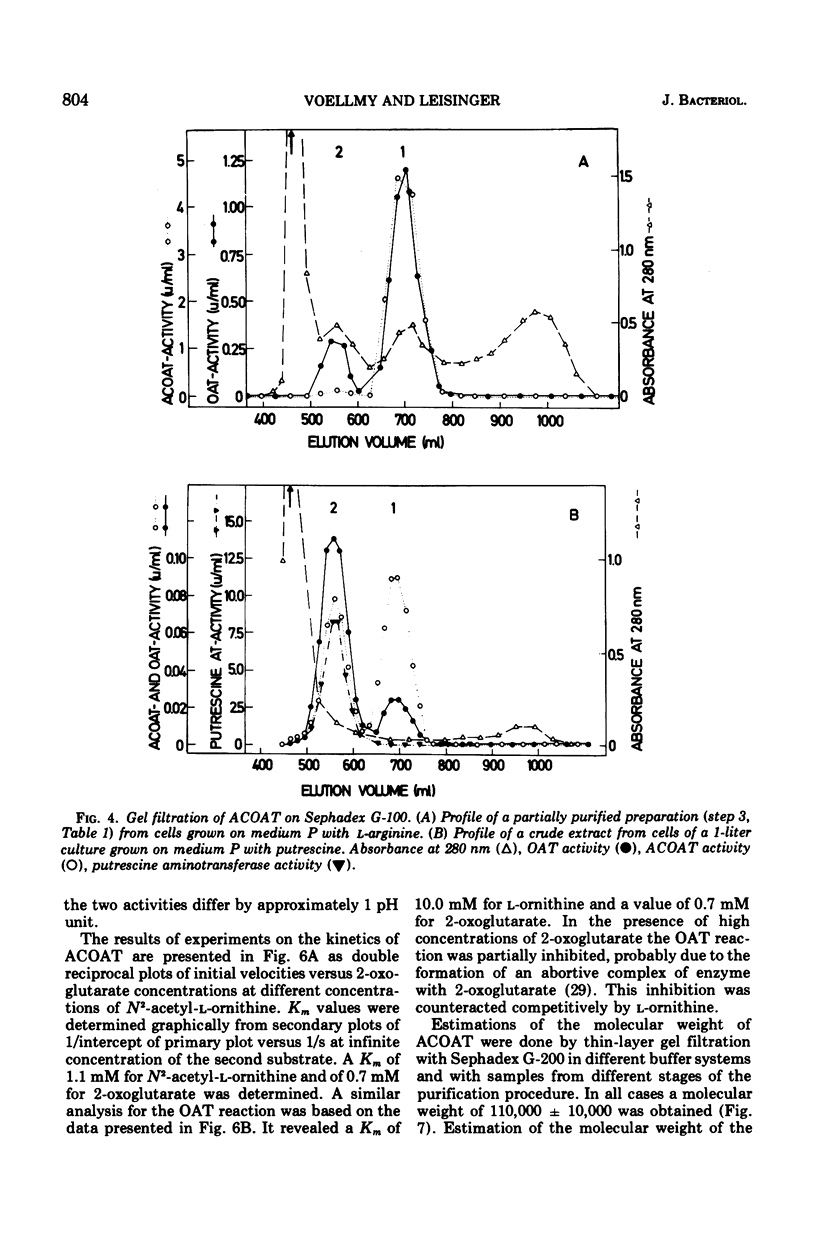
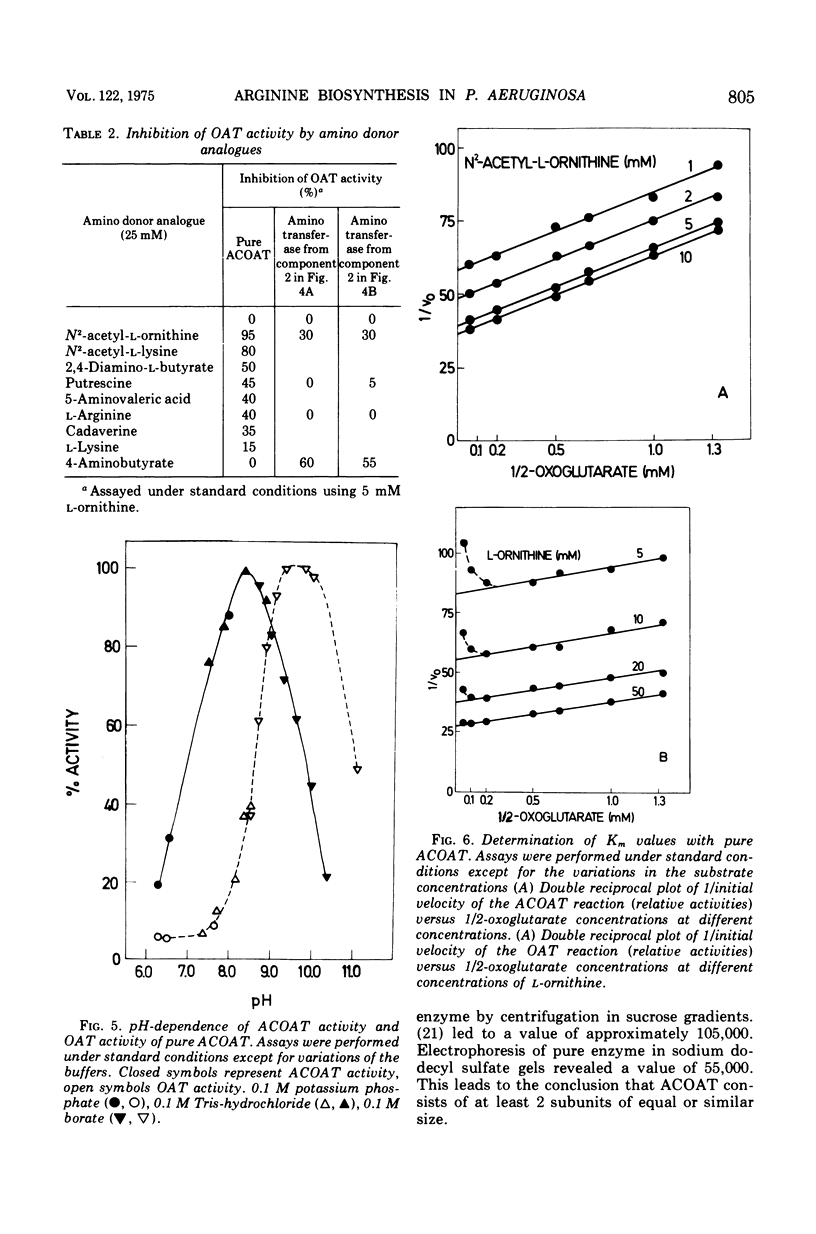
In Pseudomonas aeruginosa N-2-acetylornithine 5-aminotransferase (ACOAT), the fourth enzyme of arginine biosynthesis is induced about 15-fold by cultivating the organism on a medium with L-arginine as the sole carbon and nitrogen source. Synthesis of the enzyme is subject to catabolite repression and nitrogen source. Synthesis of the enzyme is subject to catabolite repression by a variety of carbon sources. ACOAT from strain PAO 1 was purified over 40-fold to electrophoretic homogeneity. A molecular weight of approximately 110,000 was obtained by thin-layer gel filtration. Electrophoresis in sodium dodecyl sulfate gels gave a single band corresponding to a molecular weight of 55,000. Purified ACOAT catalyzes the transamination of N-2-acetyl-L-ornithine as well as of L-ornithine with 2-oxoglutarate (Km values of 1.1, 10.0, and 0.7 mM, respectively). With N-2-acetyl-L-ornithine as amino donor, the pH-optimum of the enzymatic reaction is 8.5; with L-ornithine as amino donor, 9.5. The catalytic properties of ACOAT as well as the regulation of its synthesis indicate that in P. aeruginosa this enzyme functions in the biosynthesis as well as in the catabolism of L-arginine.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBRECHT A. M., VOGEL H. J. ACETYLORNITHINE DELTA-TRANSAMINASE. PARTIAL PURIFICATION AND REPRESSION BEHAVIOR. J Biol Chem. 1964 Jun;239:1872–1876. [PubMed] [Google Scholar]
- Bartnik E., Weglenski P. Regulation of arginine catabolism in Aspergillus nidulans. Nature. 1974 Aug 16;250(467):590–592. doi: 10.1038/250590a0. [DOI] [PubMed] [Google Scholar]
- Billheimer J. T., Jones E. E. Inducible and repressible acetylornithine delta-transaminase in Escherichia coli: different proteins. Arch Biochem Biophys. 1974 Apr 2;161(2):647–651. doi: 10.1016/0003-9861(74)90349-x. [DOI] [PubMed] [Google Scholar]
- Brohn F., Tchen T. T. A single transaminase for 1,4-diaminobutane and 4-aminobutyrate in a Pseudomonas species. Biochem Biophys Res Commun. 1971 Nov 5;45(3):578–582. doi: 10.1016/0006-291x(71)90456-6. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. III. Prediction of initial velocity and inhibition patterns by inspection. Biochim Biophys Acta. 1963 Feb 12;67:188–196. doi: 10.1016/0006-3002(63)91816-x. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Davis R. H., Mora J. Mutants of Neurospora crassa deficient in ornithine-delta-transmainase. J Bacteriol. 1968 Aug;96(2):383–388. doi: 10.1128/jb.96.2.383-388.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth G. W., Theil E. C., Jones E. E., Vogel H. J. Isolation and characterization of arginine-inducible acetylornithine delta-transaminase from Escherichia coli. J Biol Chem. 1970 Oct 25;245(20):5354–5359. [PubMed] [Google Scholar]
- GROSS D. Two-dimensional high-voltage paper electrophoresis of amino--and other organic acids. Nature. 1959 Oct 24;184:1298–1301. doi: 10.1038/1841298b0. [DOI] [PubMed] [Google Scholar]
- Haas D., Kurer V., Leisinger T. N-acetylglutamate synthetase of Pseudomonas aeruginosa. An assay in vitro and feedback inhibition by arginine. Eur J Biochem. 1972 Dec 4;31(2):290–295. doi: 10.1111/j.1432-1033.1972.tb02531.x. [DOI] [PubMed] [Google Scholar]
- Holloway B. W., Krishnapillai V., Stanisich V. Pseudomonas genetics. Annu Rev Genet. 1971;5:425–446. doi: 10.1146/annurev.ge.05.120171.002233. [DOI] [PubMed] [Google Scholar]
- Isaac J. H., Holloway B. W. Control of arginine biosynthesis in Pseudomonas aeruginosa. J Gen Microbiol. 1972 Dec;73(3):427–438. doi: 10.1099/00221287-73-3-427. [DOI] [PubMed] [Google Scholar]
- JAKOBY W. B., FREDERICKS J. Pyrrolidine and putrescine metabolism: gamma-aminobutyraldehyde dehydrogenase. J Biol Chem. 1959 Aug;234(8):2145–2150. [PubMed] [Google Scholar]
- Kakimoto T., Shibatani T., Chibata I. Crystallization of L-arginine deiminase from Pseudomonas Putida. FEBS Lett. 1971 Dec 1;19(2):166–168. doi: 10.1016/0014-5793(71)80505-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LYSENKO O. Pseudomonas--an attempt at a general classification. J Gen Microbiol. 1961 Jul;25:379–408. doi: 10.1099/00221287-25-3-379. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- MORRIS C. J. THIN-LAYER CHROMATOGRAPHY OF PROTEINS ON SEPHADEX G-100 AND G-200. J Chromatogr. 1964 Oct;16:167–175. doi: 10.1016/s0021-9673(01)82451-1. [DOI] [PubMed] [Google Scholar]
- Middelhoven W. J. Induction and repression of arginase and ornithine transaminase in baker's yeast. Antonie Van Leeuwenhoek. 1970;36(1):1–19. doi: 10.1007/BF02069003. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Rodwell V. W. Metabolism of basic amino acids in Pseudomonas putida. Intermediates in L-arginine catabolism. J Biol Chem. 1971 Aug 25;246(16):5053–5058. [PubMed] [Google Scholar]
- Radola B. J. Thin-layer gel filtration of proteins. I. Method. J Chromatogr. 1968 Nov 5;38(1):61–77. doi: 10.1016/0021-9673(68)85009-5. [DOI] [PubMed] [Google Scholar]
- SHOESMITH J. H., SHERRIS J. C. Studies on the mechanism of arginine-activated motility in a Pseudomonas strain. J Gen Microbiol. 1960 Feb;22:10–24. doi: 10.1099/00221287-22-1-10. [DOI] [PubMed] [Google Scholar]
- Udaka S. Pathway-specific pattern of control of arginine biosynthesis in bacteria. J Bacteriol. 1966 Feb;91(2):617–621. doi: 10.1128/jb.91.2.617-621.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voellmy R., Leisinger T. Induktion der N2-Acetylornithin-5-aminotransferase aus Pseudomonas aeruginosa durch Arginin. Pathol Microbiol (Basel) 1974;41(3-4):188–190. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



