Abstract
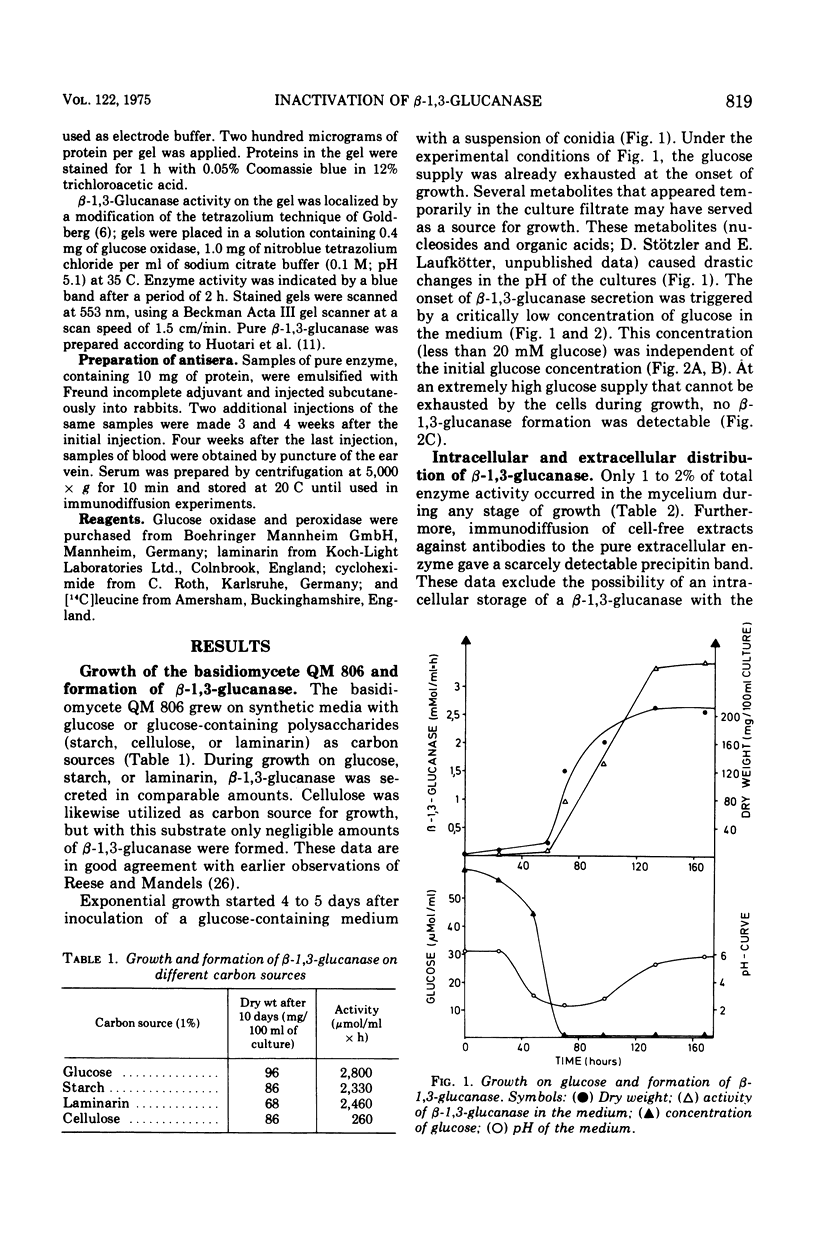
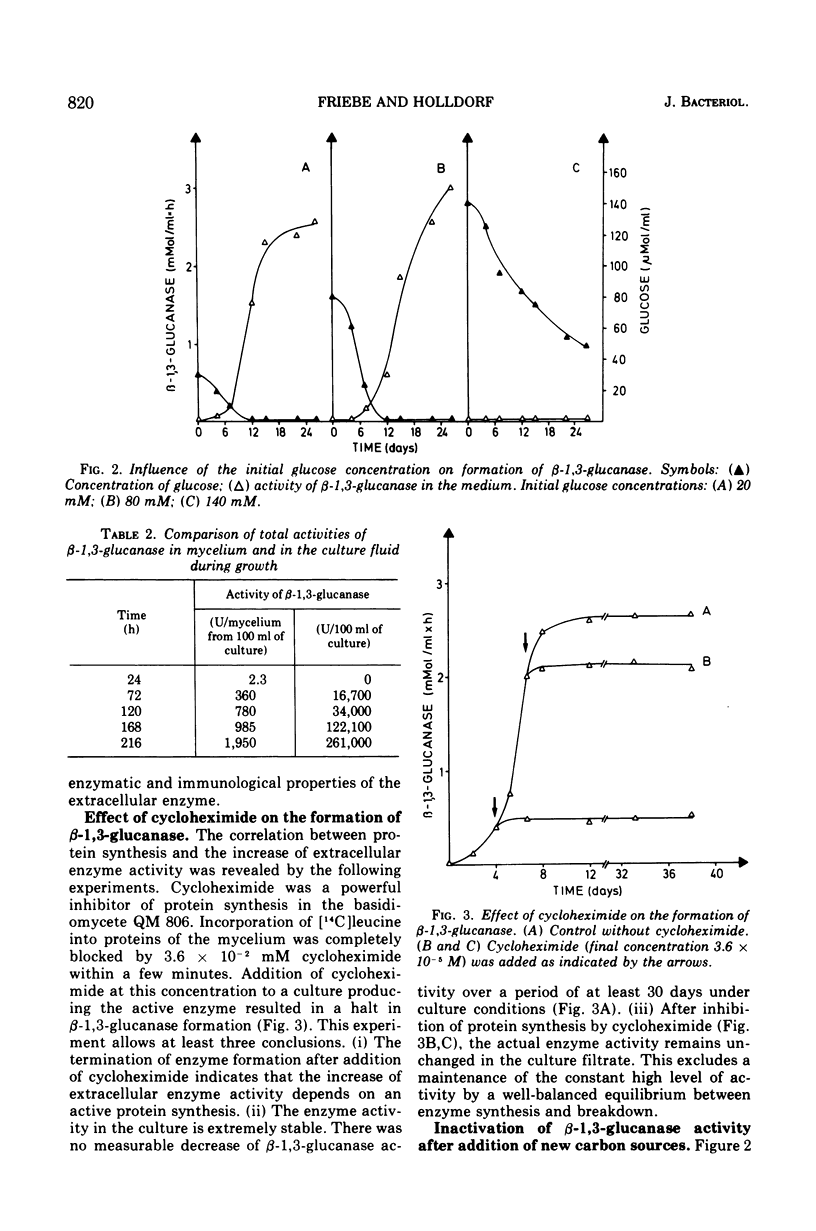
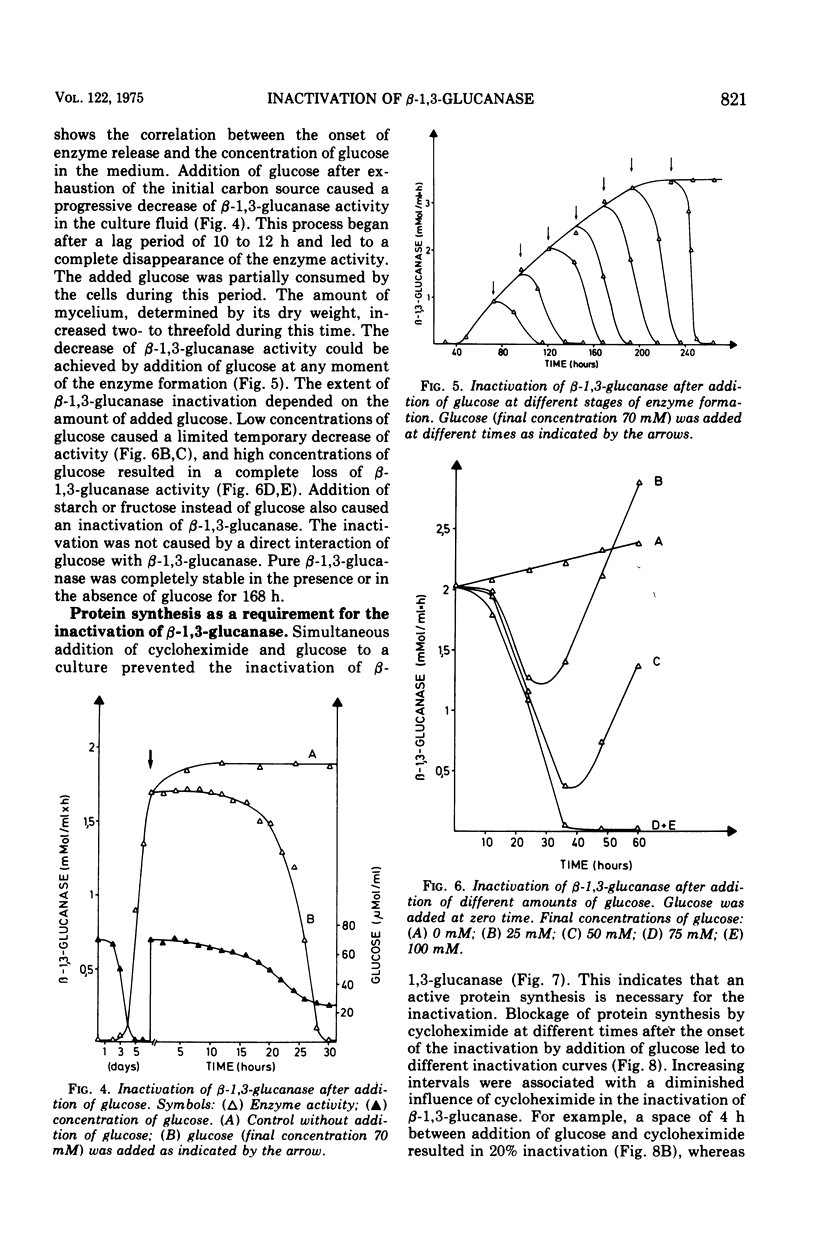
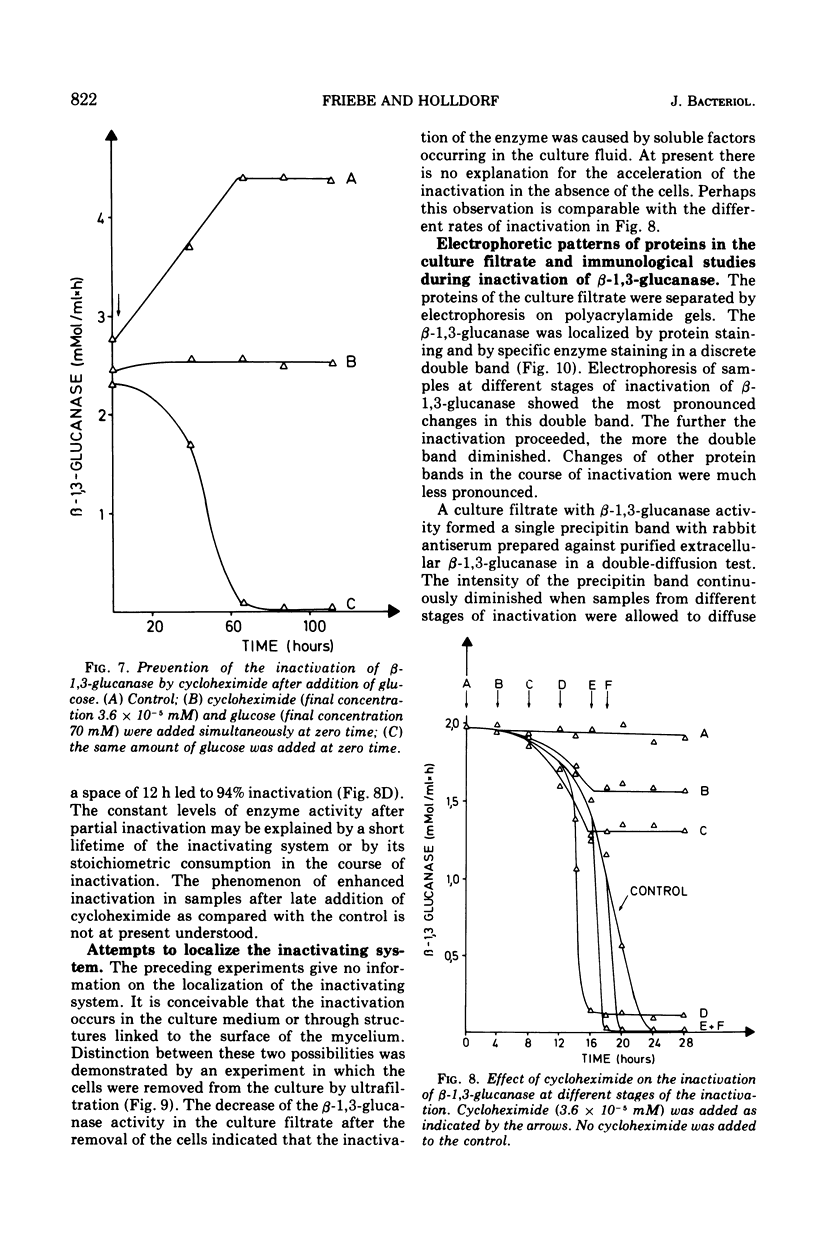
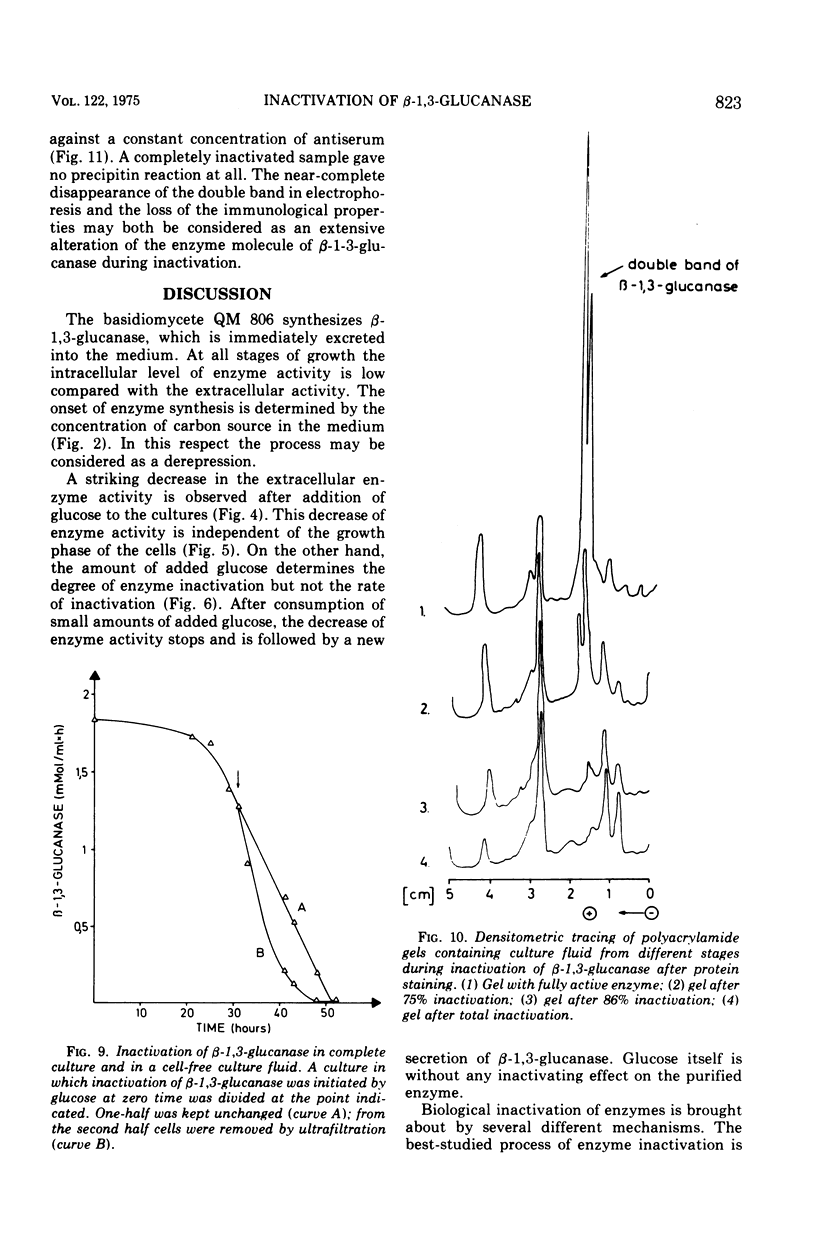
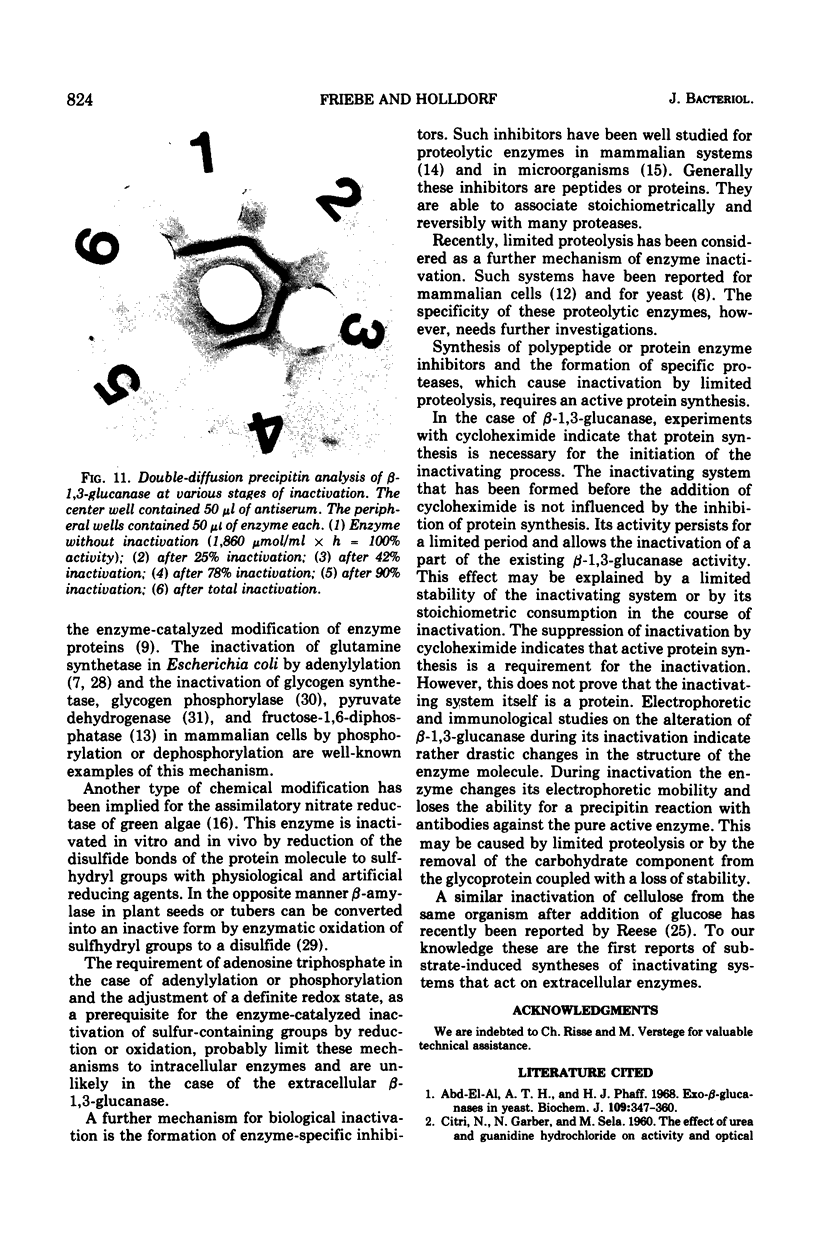
The basidiomycete QM 806 excreted large amounts of beta-1,3-glucanase into the culture medium. Synthesis and excretion of the enzyme were triggered by a critically low concentration of carbon source. The extracellular beta-1,3-glucanase exhibited a remarkable stability. Addition of glucose or other carbon sources to a culture after consumption of the initial carbon source led to an inactivation of the extracellular beta-1,3-glucanase by an inactivating system, which could be separated from the cells. The inactivation of beta-1,3-glucanse was prevented by cycloheximide. This indicates the necessity of active protein synthesis for the inactivation process but does not prove that the inactivating system itself is a protein. Marked changes in the electrophoretic mobility and immunological properties of beta-1,3-glucanase indicate rather profound alterations of the enzyme protein in the course of inactivation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abd-el-Al A. T., Phaff H. J. Exo-beta-glucanases in yeast. Biochem J. 1968 Sep;109(3):347–360. doi: 10.1042/bj1090347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CITRI N., GARBER N., SELA M. The effect of urea and guanidine hydrochloride on activity and optical rotation of penicillinase. J Biol Chem. 1960 Dec;235:3454–3459. [PubMed] [Google Scholar]
- Comp P. C., Lester G. Properties of an extracellular -galactosidase secreted by Neurospora crassa. J Bacteriol. 1971 Jul;107(1):162–167. doi: 10.1128/jb.107.1.162-167.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascón S., Neumann N. P., Lampen J. O. Comparative study of the properties of the purified internal and external invertases from yeast. J Biol Chem. 1968 Apr 10;243(7):1573–1577. [PubMed] [Google Scholar]
- Goldberg E. Lactic and Malic Dehydrogenases in Human Spermatozoa. Science. 1963 Feb 15;139(3555):602–603. doi: 10.1126/science.139.3555.602. [DOI] [PubMed] [Google Scholar]
- Holzer H., Duntze W. Metabolic regulation by chemical modification of enzymes. Annu Rev Biochem. 1971;40:345–374. doi: 10.1146/annurev.bi.40.070171.002021. [DOI] [PubMed] [Google Scholar]
- Holzer H. Regulation of enzymes by enzyme-catalyzed chemical modification. Adv Enzymol Relat Areas Mol Biol. 1969;32:297–326. doi: 10.1002/9780470122778.ch7. [DOI] [PubMed] [Google Scholar]
- Hulme M. A., Stranks D. W. Induction and the regulation of production of cellulase by fungi. Nature. 1970 May 2;226(5244):469–470. doi: 10.1038/226469a0. [DOI] [PubMed] [Google Scholar]
- Huotari F. I., Nelson T. E., Smith F., Kirkwood S. Purification of an exo-beta-D-(1 bonded to 3)-glucanase from Basidiomycete species QM 806. J Biol Chem. 1968 Mar 10;243(5):952–956. [PubMed] [Google Scholar]
- Kratowich N., Mendicino J. Role of enzyme-enzyme interactions in the regulation of gluconeogenesis. Effect of fatty acids, tricarboxylic acid cycle intermediates, and dinitrophenol on the rate of inactivation on D-frucose, 1,6-diphosphatase by kidney mitochondria. J Biol Chem. 1970 May 25;245(10):2483–2492. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lenney J. F., Dalbec J. M. Yeast proteinase B: identification of the inactive form as an enzyme-inhibitor complex. Arch Biochem Biophys. 1969 Jan;129(1):407–409. doi: 10.1016/0003-9861(69)90194-5. [DOI] [PubMed] [Google Scholar]
- MANDELS M., PARRISH F. W., REESE E. T. Sophorose as an inducer of cellulase in Trichoderma viride. J Bacteriol. 1962 Feb;83:400–408. doi: 10.1128/jb.83.2.400-408.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDELS M., REESE E. T. Induction of cellulase in fungi by cellobiose. J Bacteriol. 1960 Jun;79:816–826. doi: 10.1128/jb.79.6.816-826.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M. G., Eberhart B. Regulation of cellulase and cellobiase in Neurospora crassa. Biochem Biophys Res Commun. 1966 Sep 8;24(5):782–785. doi: 10.1016/0006-291x(66)90394-9. [DOI] [PubMed] [Google Scholar]
- Nelson T. E., Johnson J., Jr, Jantzen E., Kirkwood S. Action pattern and specificity of an exo-beta-(1--3)-D-glucanase from basidiomycetes species QM 806. J Biol Chem. 1969 Nov 10;244(21):5972–5980. [PubMed] [Google Scholar]
- POLLOCK M. R., RICHMOND M. H. Low cyst(e)ine content of bacterial extracellular proteins: its possible physiological significance. Nature. 1962 May 5;194:446–449. doi: 10.1038/194446a0. [DOI] [PubMed] [Google Scholar]
- Pazur J. H., Simpson D. L., Knull H. R. Biosynthesis of glucohydrolase I, a glycoenzyme from Aspergillus niger. Biochem Biophys Res Commun. 1969 Aug 7;36(3):394–400. doi: 10.1016/0006-291x(69)90577-4. [DOI] [PubMed] [Google Scholar]
- REESE E. T., MANDELS M. Beta-D-1, 3 Glucanases in fungi. Can J Microbiol. 1959 Apr;5(2):173–185. doi: 10.1139/m59-022. [DOI] [PubMed] [Google Scholar]
- REESE E. T., MANDELS M. Use of enzymes in isolation and analysis of polysaccharides. Appl Microbiol. 1959 Nov;7:378–387. doi: 10.1128/am.7.6.378-387.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro B. M., Kingdon H. S., Stadtman E. R. Regulation of glutamine synthetase. VII. Adenylyl glutamine synthetase: a new form of the enzyme with altered regulatory and kinetic properties. Proc Natl Acad Sci U S A. 1967 Aug;58(2):642–649. doi: 10.1073/pnas.58.2.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradlin J., Thoma J. A. Beta-amylase thiol groups. Possible regulator sites. J Biol Chem. 1970 Jan 10;245(1):117–127. [PubMed] [Google Scholar]
- Villar-Palasi C., Larner J. Glycogen metabolism and glycolytic enzymes. Annu Rev Biochem. 1970;39:639–672. doi: 10.1146/annurev.bi.39.070170.003231. [DOI] [PubMed] [Google Scholar]
- Wieland O., Jagow-Westermann B. v. ATP-dependent inactivation of heart muscle pyruvate dehydrogenase and reactivation by Mg(++). FEBS Lett. 1969 Jun;3(4):271–274. doi: 10.1016/0014-5793(69)80156-0. [DOI] [PubMed] [Google Scholar]