Abstract
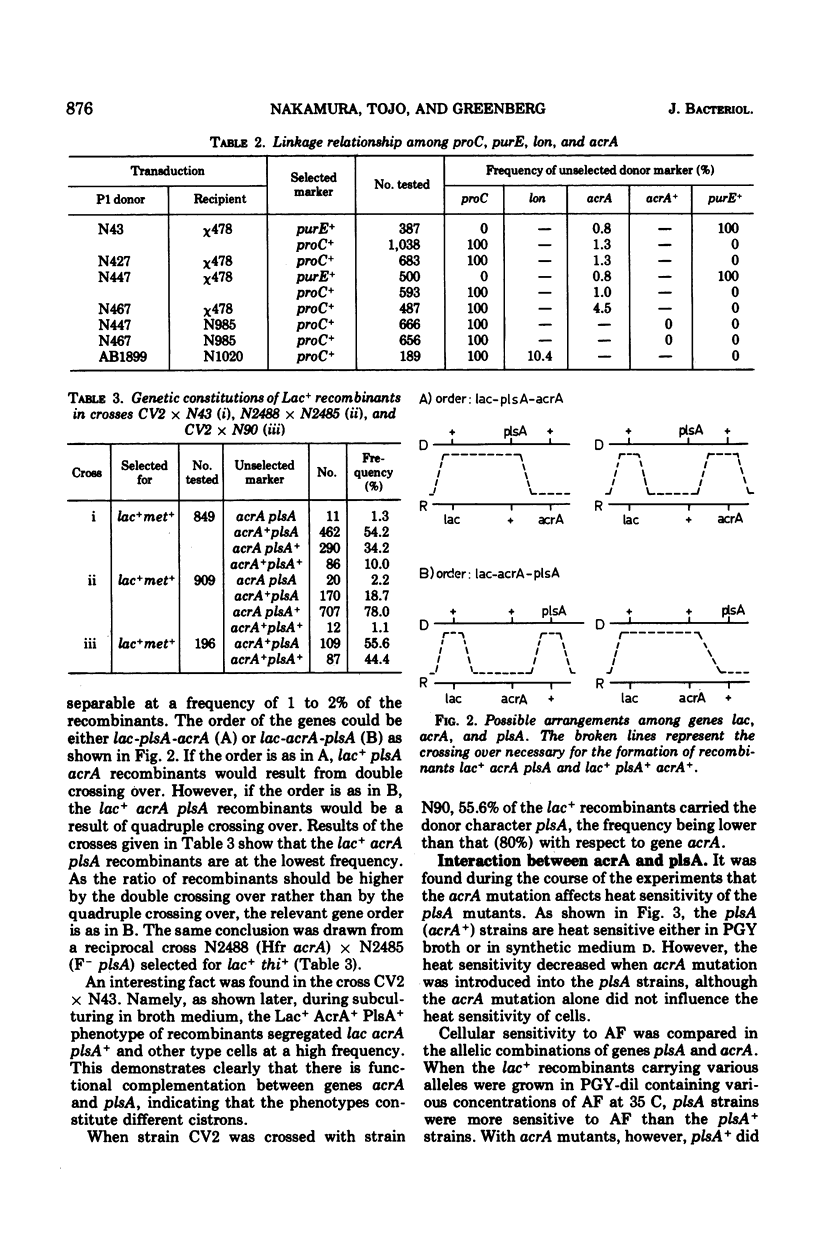
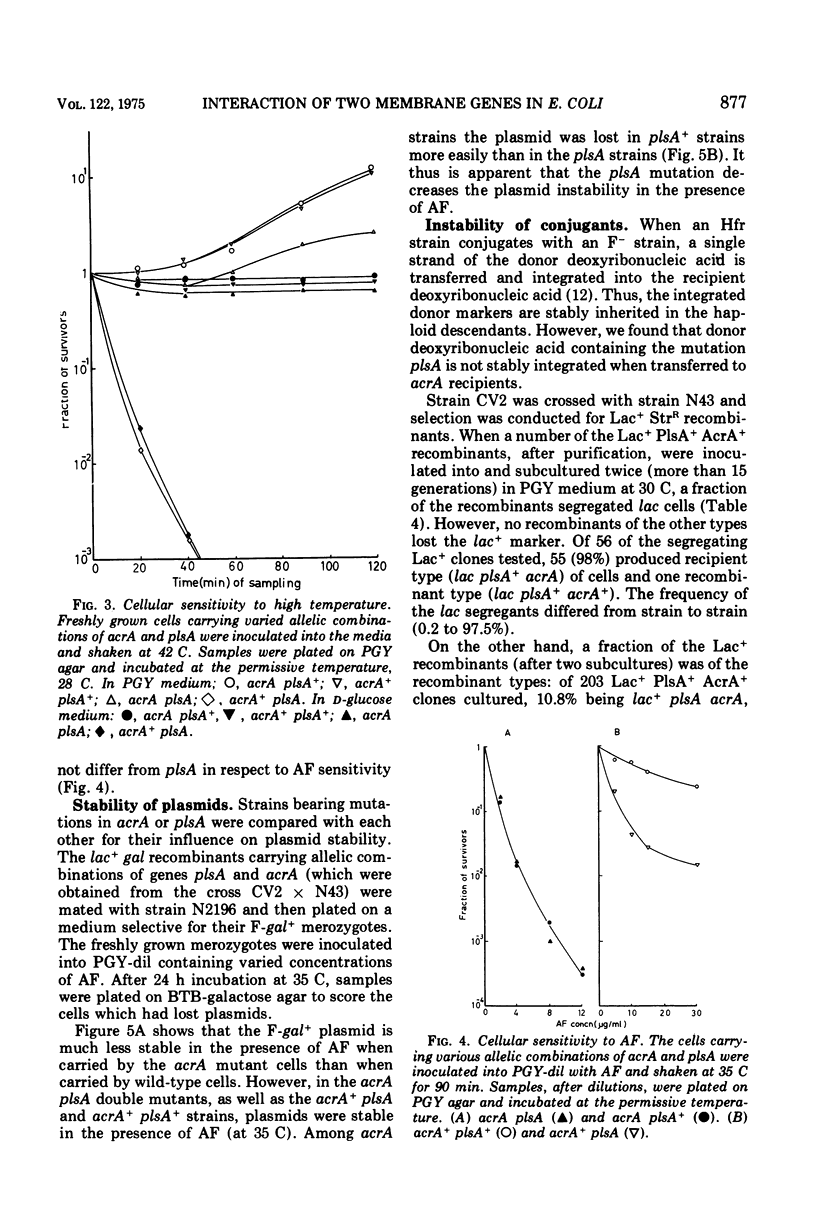
The mutation acrA1, leading to acriflavine sensitivity through disorganization of the plasma membrane, is located between proC and purE on the Escherichia coli K-12 chromosome. Gene plsA has been reported to determine biosynthesis of membrane phospholipid and to be located very near acrA (1). Genes acrA and plsA fall into different cistrons and are arranged in the order proC-acrA-plasA-purE. The genes were shown to interact with each other. Introduction of acrA mutation into a plsA temperature-sensitive mutant mitigated the heat sensitivity. Plasmid (F-gal+) stability in acrA mutants was restored by introduction of the plasA mutation into the acrA cells. When an Hfr plsA donor was conjugated with an acrA recipient, or when reciprocally conjugated, the exogenotes were eliminated at high frequency during subsequent subcultivation in broth. However, the exogenotes were not eliminated in all other allelic combinations of genes acrA and plsA. When an F-gal+ plasmid was introduced into the unstable heterozygotes (acrA+plsA/acrApls1+), the plasmids were stably hosted, whereas the acrA+ plasA exogenotes were spontaneously lost at a high frequency. On the other hand, when the unstable heterozygotes carrying F-gal+ were cultured in acriflavine-containing medium, the F-gal+ plasmids were preferentially eliminated but the acrA+plasA exogenotes were not affected. The results suggest that the organization of the plasma membrane controls the recombination of the exogenotes introduced into zygotes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cronan J. E., Jr, Godson G. N. Mutants of Escherichia coli with temperature-sensitive lesions in membrane phospholipid synthesis: genetic analysis of glycerol-3-phosphate acyltransferase mutants. Mol Gen Genet. 1972;116(3):199–210. doi: 10.1007/BF00269765. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Ray T. K., Vagelos P. R. Selection and characterization of an E. coli mutant defective in membrane lipid biosynthesis. Proc Natl Acad Sci U S A. 1970 Mar;65(3):737–744. doi: 10.1073/pnas.65.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donch J., Greenberg J. Ultraviolet sensitivity gene of Escherichia coli B. J Bacteriol. 1968 May;95(5):1555–1559. doi: 10.1128/jb.95.5.1555-1559.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Nakamura H. Gene-Controlled Resistance to Acriflavine and Other Basic Dyes in Escherichia coli. J Bacteriol. 1965 Jul;90(1):8–14. doi: 10.1128/jb.90.1.8-14.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H. Genetic determination of resistance to acriflavine, phenethyl alcohol, and sodium dodecyl sulfate in Escherichia coli. J Bacteriol. 1968 Oct;96(4):987–996. doi: 10.1128/jb.96.4.987-996.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H. Phenethyl alcohol sensitivity in Escherichia coli. J Bacteriol. 1967 Mar;93(3):1183–1184. doi: 10.1128/jb.93.3.1183-1184.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H., Suganuma A. Membrane mutation associated with sensitivity to acriflavine in Escherichia coli. J Bacteriol. 1972 Apr;110(1):329–335. doi: 10.1128/jb.110.1.329-335.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray T. K., Cronan J. E., Jr, Mavis R. D., Vagelos P. R. The specific acylation of glycerol 3-phosphate to monoacylglycerol 3-phosphate in Escherichia coli. Evidence for a single enzyme conferring this specificity. J Biol Chem. 1970 Dec 10;245(23):6442–6448. [PubMed] [Google Scholar]
- Siddiqi O., Fox M. S. Integration of donor DNA in bacterial conjugation. J Mol Biol. 1973 Jun 15;77(1):101–123. doi: 10.1016/0022-2836(73)90365-3. [DOI] [PubMed] [Google Scholar]
- Sugino Y. Mutants of Escherichia coli sensitive to methylene blue and acridines. Genet Res. 1966 Feb;7(1):1–11. doi: 10.1017/s0016672300009423. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


