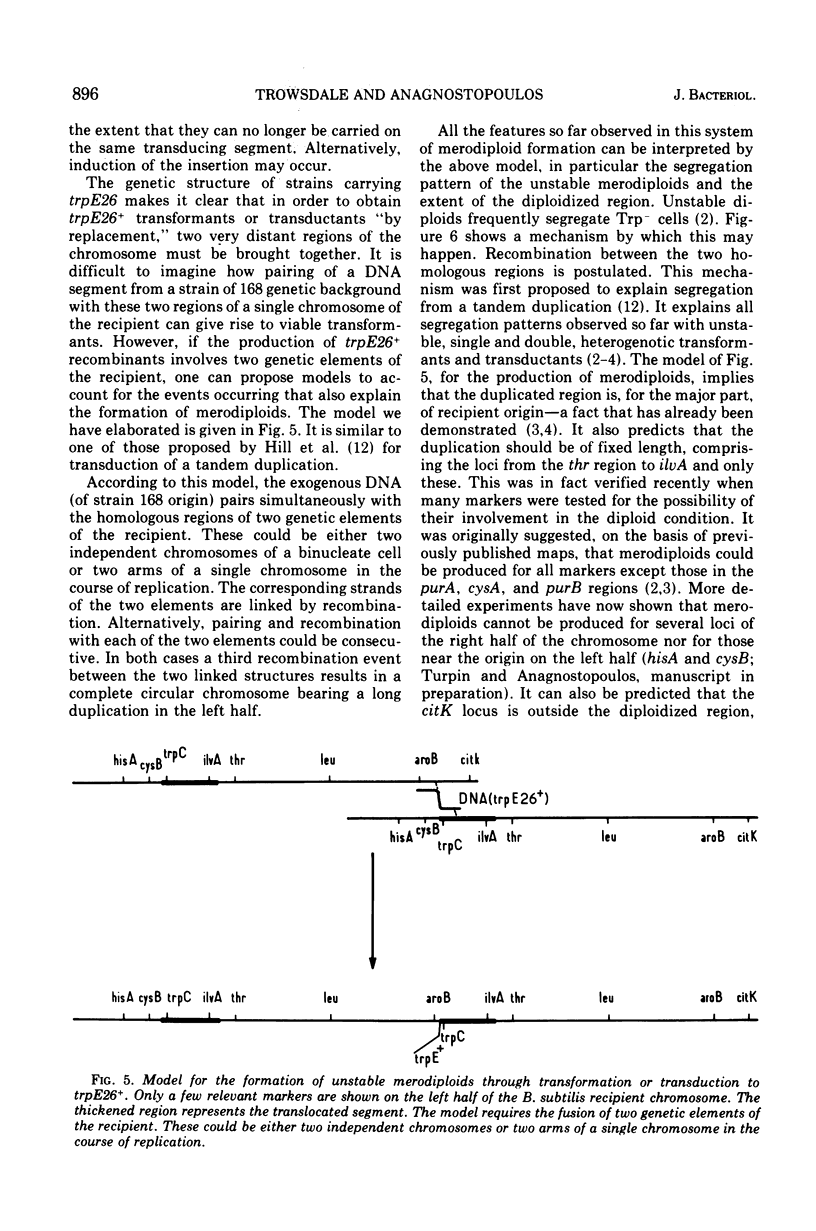
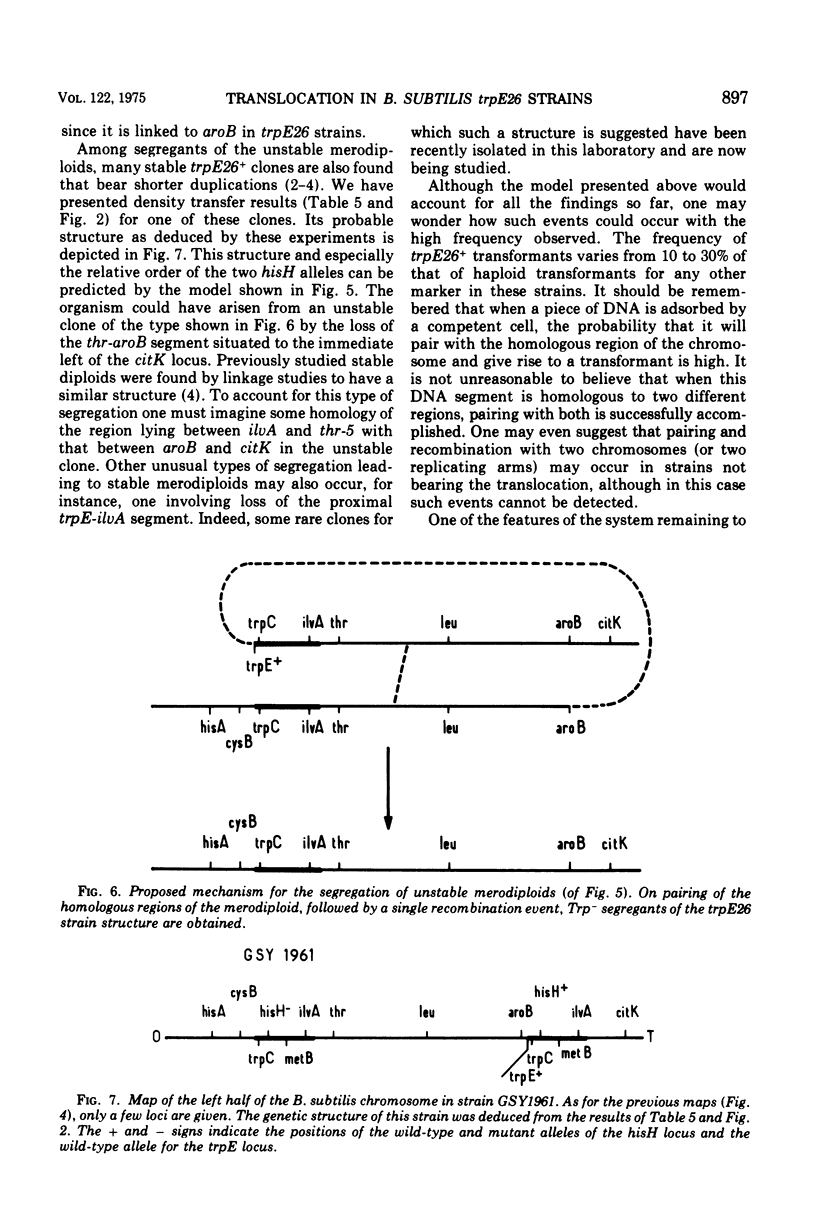
Abstract
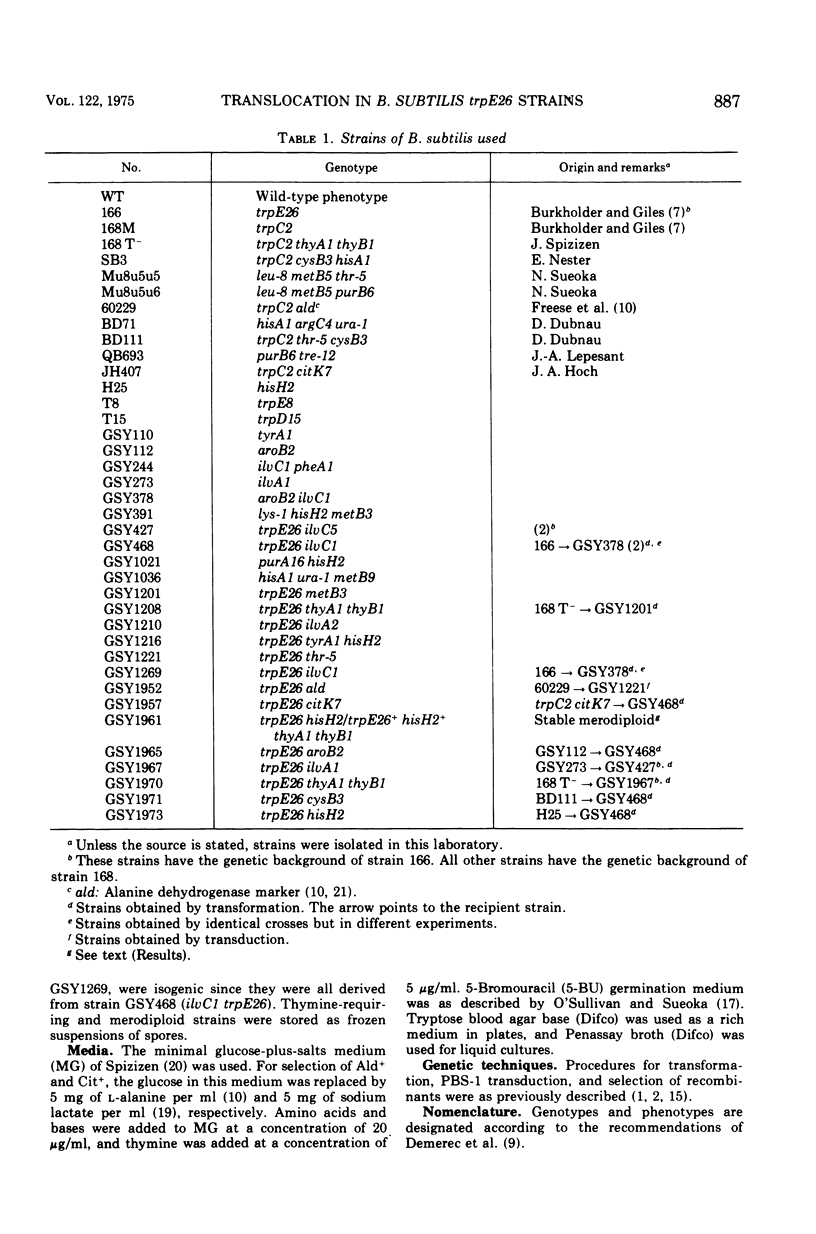
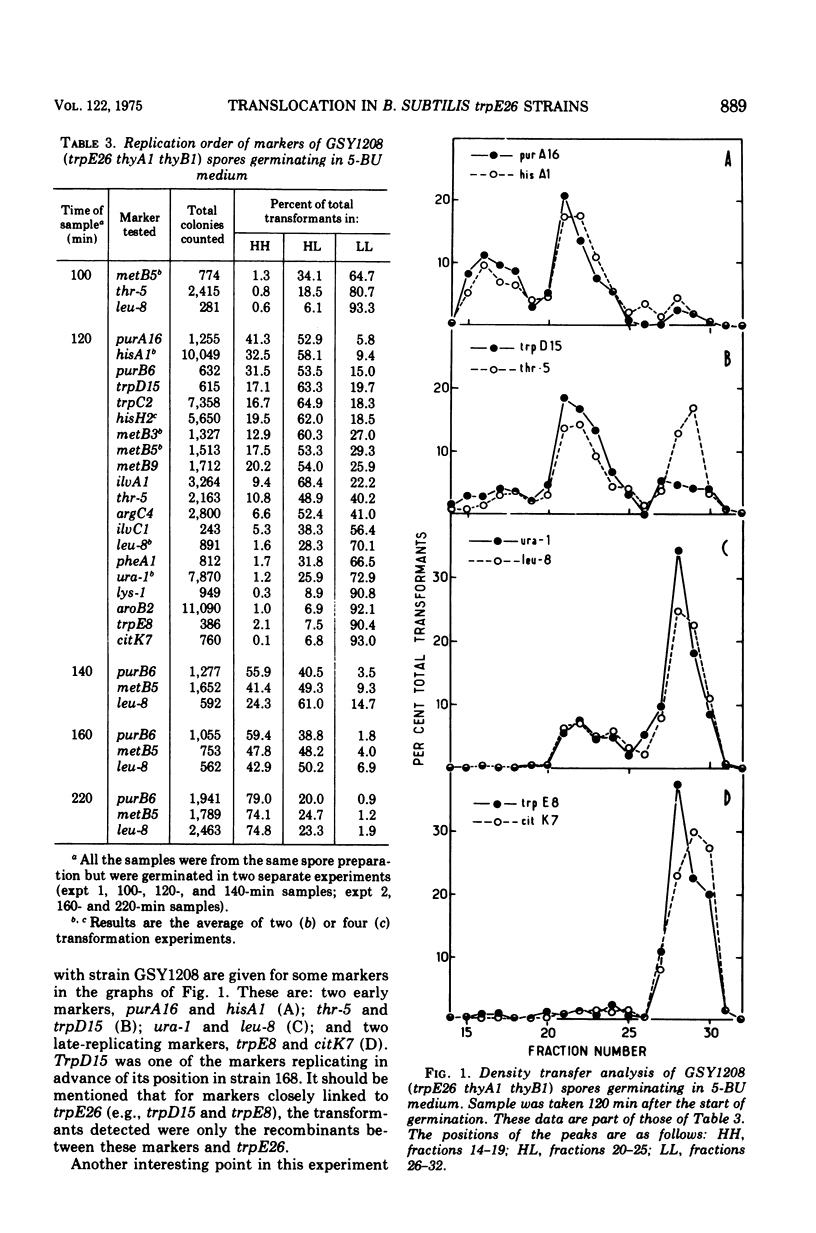
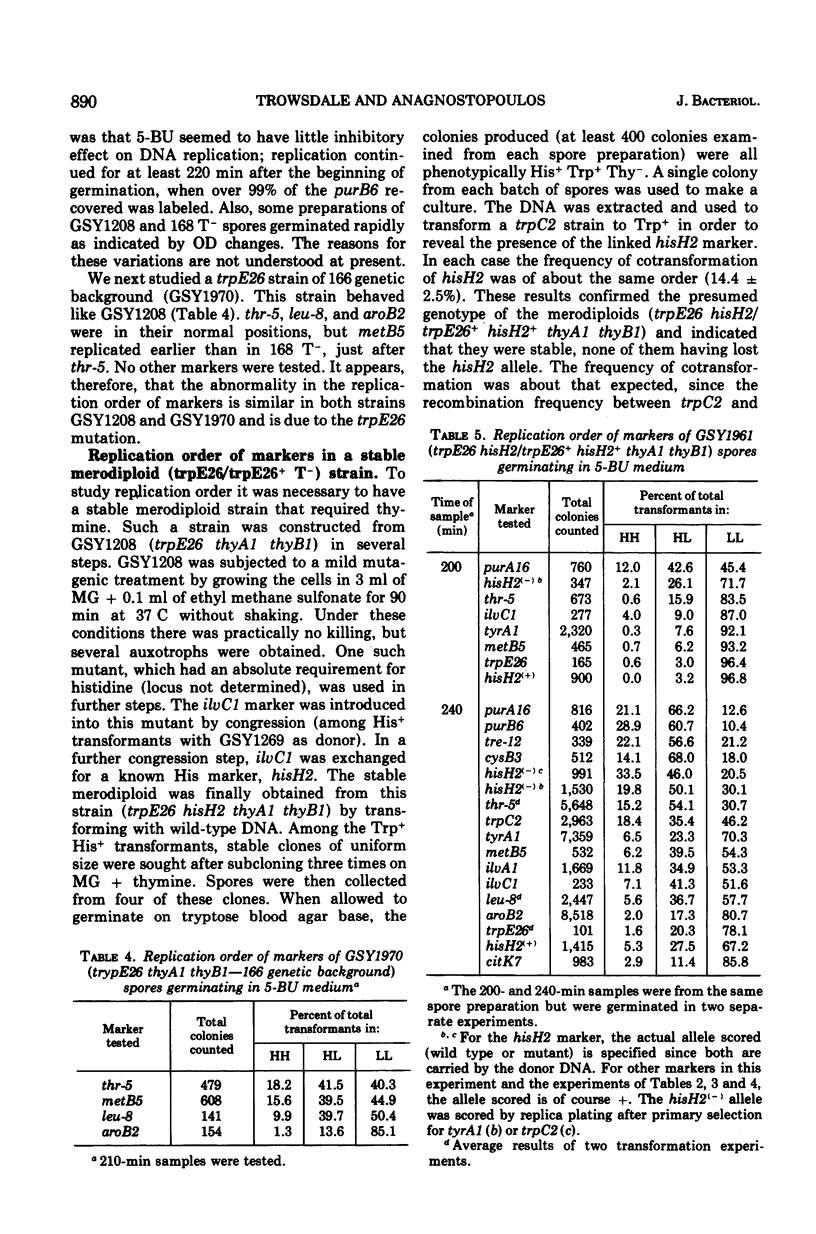
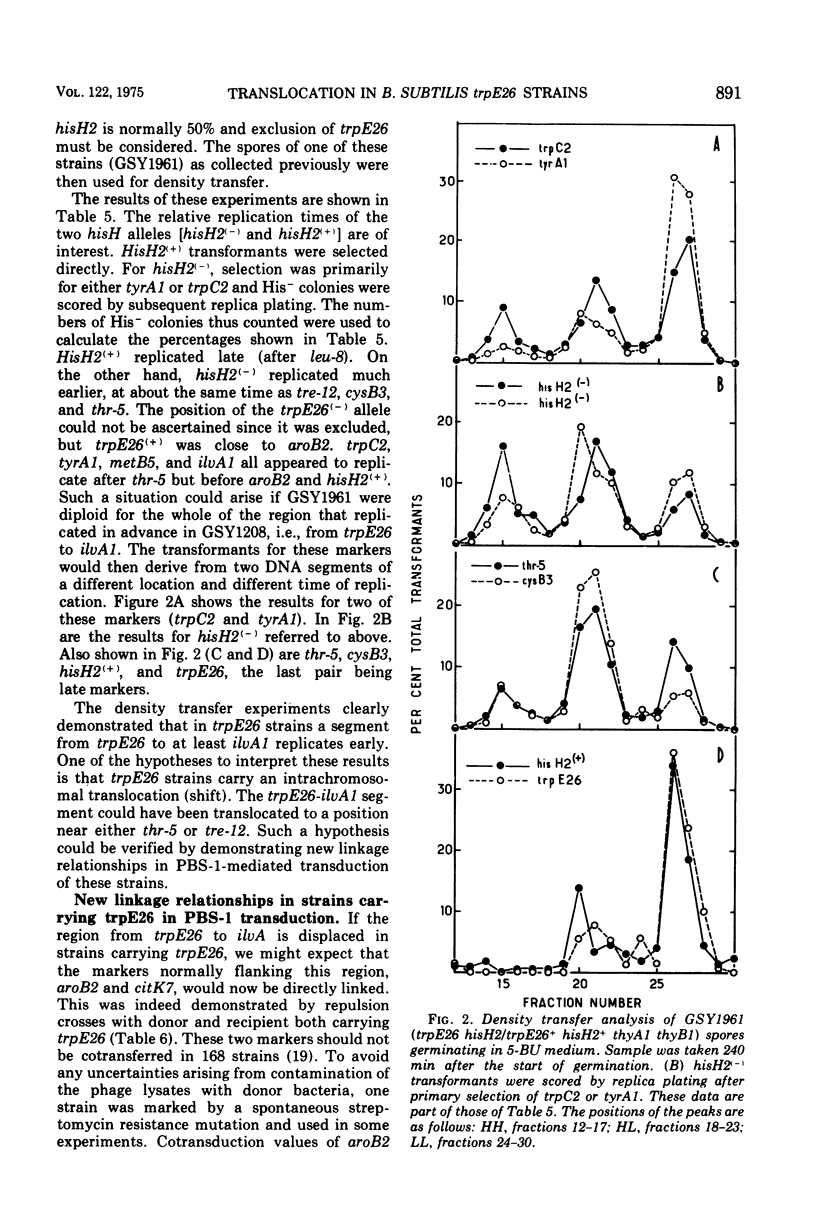
The replication order of markers was studied in Bacillus subtilis strains bearing the trpE26 mutation by the use of the density transfer technique. An important difference in this order was observed in comparison with that of strain 168 T-. All markers tested of a chromosome segment extending from trpD to ilvA replicated early, after purB6 and before thr-5. Two markers flanking this region, trpE8 and citK7, replicated late as usual. The results suggested that this segment was shifted in trpE26 strains to a region closer to the origin of replication. PBS-1-mediated transduction crosses corroborated this hypothesis and revealed the position of the translocated segment. (i) Linkage was demonstrated for markers in the segment (hisH2, tryA1, met B3, ilvA2) to thr-5 and ald; (ii) aroB2 and citK7 were found to be linked; and (iii) linkage of cysB3 to thr-5 was lost in trpE26 strains. These findings made it possible to account for the characteristics of the trpE26 mutation and to propose a model explaining the fact that all trpE26+ transformants or transductants are merodiploid. The model calls for fusion of two genetic elements: two independent chromosomes, or two arms of a replicating structure. The resulting chromosome bears a long tandem duplication. Most of the features of this system of merodiploid formation can be interpreted by use of this model: the segregation pattern of the diploids, the stabilization of the unstable clones, and the length of the duplicated region. A relatively stable diploid strain was also studied by the density transfer technique. The data show that it remained diploid for the region corresponding to the translocated segment and are in agreement with the structure predicted by the model.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audit C., Anagnostopoulos C. Genetic studies relating to the production of transformed clones diploid in the tryptophan region of the Bacillus subtilis genome. J Bacteriol. 1973 Apr;114(1):18–27. doi: 10.1128/jb.114.1.18-27.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer H. P., Wermundsen I. E., Austrian R. Qualitative differences in the behavior of pneumoncoccal deoxyribonucleic acids transforming to the same capsular type. J Bacteriol. 1967 Jan;93(1):320–333. doi: 10.1128/jb.93.1.320-333.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer H. P., Wermundsen I. E. Unstable binary capsulated transformants in pneumococcus. J Bacteriol. 1969 Jun;98(3):1073–1079. doi: 10.1128/jb.98.3.1073-1079.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS S. R., 3rd A STABLE PARTIAL DIPLOID STRAIN OF ESCHERICHIA COLI. Genetics. 1964 Oct;50:679–694. doi: 10.1093/genetics/50.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREESE E., PARK S. W., CASHEL M. THE DEVELOPMENTAL SIGNIFICANCE OF ALANINE DEHYDROGENASE IN BACILLUS SUBTILIS. Proc Natl Acad Sci U S A. 1964 Jun;51:1164–1172. doi: 10.1073/pnas.51.6.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harford N. Bidirectional chromosome replication in Bacillus subtilis 168. J Bacteriol. 1975 Mar;121(3):835–847. doi: 10.1128/jb.121.3.835-847.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C. W., Schiffer D., Berg P. Transduction of merodiploidy: induced duplication of recipient genes. J Bacteriol. 1969 Jul;99(1):274–278. doi: 10.1128/jb.99.1.274-278.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamet C., Anagnostopoulos C. Etude d'une mutation très faiblement transformable au locus de la thréonine désaminase de Bacillus subtilis. Mol Gen Genet. 1969;105(3):225–242. doi: 10.1007/BF00337474. [DOI] [PubMed] [Google Scholar]
- Lepesant-Kejzlarová J., Lepesant J. A., Walle J., Billault A., Dedonder R. Revision of the linkage map of Bacillus subtilis 168: indications for circularity of the chromosome. J Bacteriol. 1975 Mar;121(3):823–834. doi: 10.1128/jb.121.3.823-834.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan A., Sueoka N. Sequential replication of the Bacillus subtilis chromosome. IV. Genetic mapping by density transfer experiment. J Mol Biol. 1967 Jul 28;27(2):349–368. doi: 10.1016/0022-2836(67)90025-3. [DOI] [PubMed] [Google Scholar]
- Ravin A. W., Takahashi E. A. Merodiploid ribosomal loci arising by transformation and mutation in pneumococcus. J Bacteriol. 1970 Jan;101(1):38–52. doi: 10.1128/jb.101.1.38-52.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutberg B., Hoch J. A. Citric acid cycle: gene-enzyme relationships in Bacillus subtilis. J Bacteriol. 1970 Nov;104(2):826–833. doi: 10.1128/jb.104.2.826-833.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIKAWA H., SUEOKA N. Sequential replication of the Bacillus subtilis chromosome. II. Isotopic transfer experiments. Proc Natl Acad Sci U S A. 1963 Jun;49:806–813. doi: 10.1073/pnas.49.6.806. [DOI] [PMC free article] [PubMed] [Google Scholar]



