Abstract
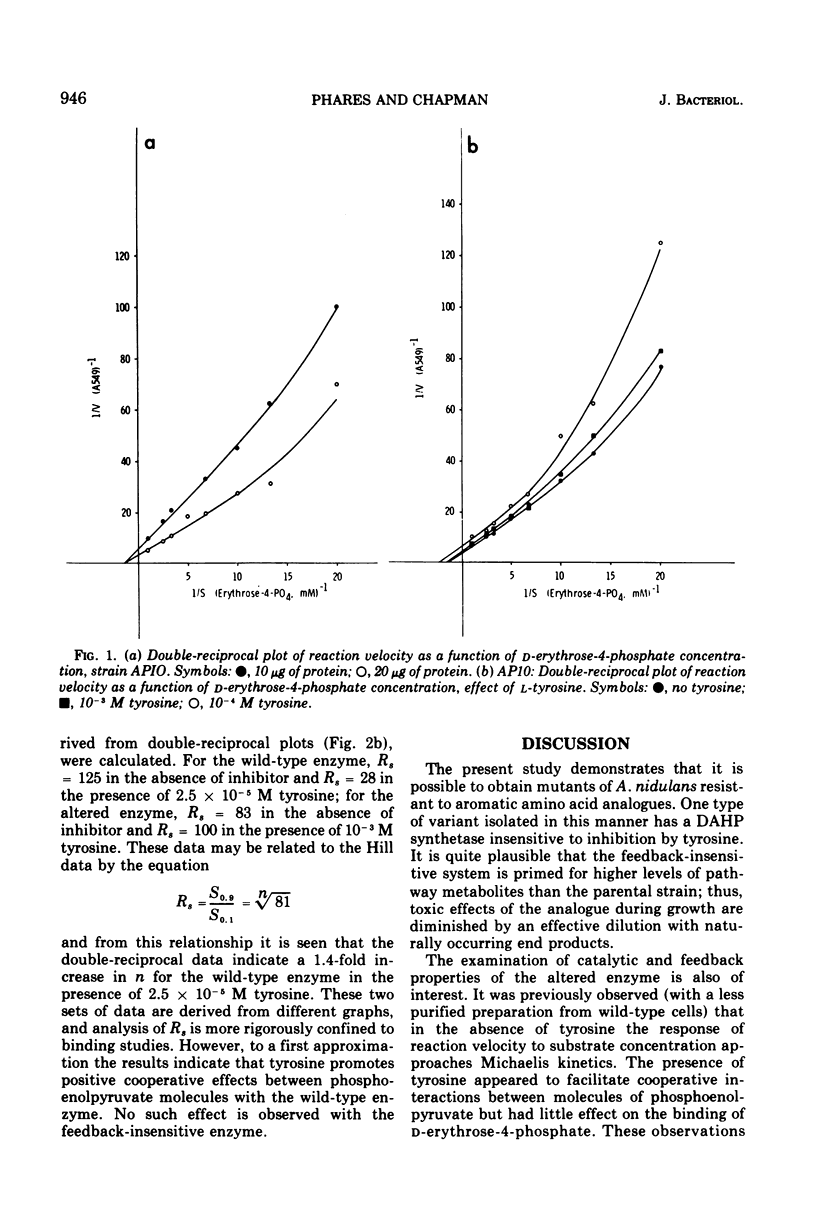
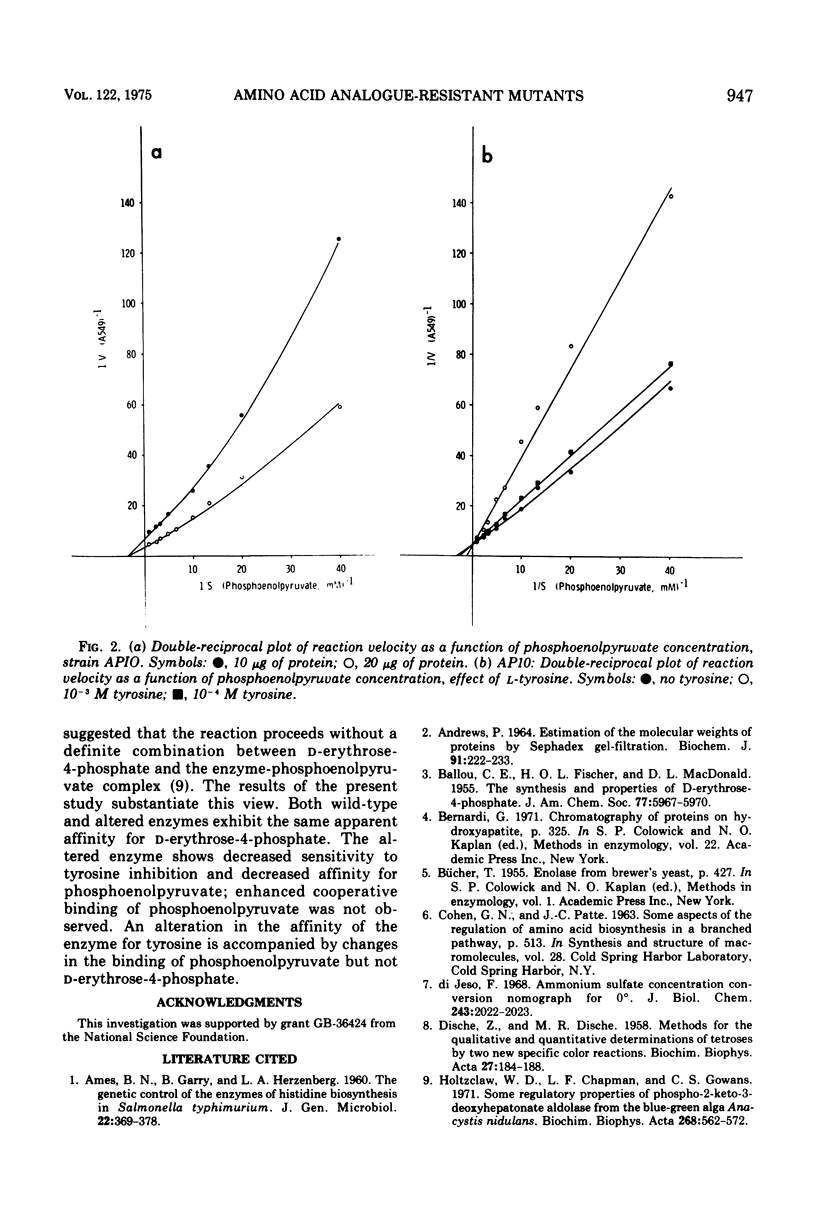
Three classes of mutants of Anacystis nidulans were selected on the basis of resistance to fluorophenylalanine and 2-amino-3-phenylbutanoic acid. The most frequent type exhibited DAHP synthetase (7-phospho-2-keto-3-deoxy-D-arabino-heptonate-D-erythrose-4-phosphate-lyase [pyruvate phosphorylating], EC 4.1.2.15) activity identical to that of the parental strain. The second type was characterized by extremely low levels of the activity. The third type had a DAHP synthetase showing decreased sensitivity to inhibition by L-tyrosine. The enzyme was purified 140-fold from wild-type and feedback-insensitive strains, and the kinetics of the reaction was examined. The activity of the wild-type enzyme was inhibited 75% in the presence of 2.0 X 10-3 M tyrosine, and the altered enzyme was inhibited 10%. The following apparent constants were obtained from kinetic studies with partially purified wild-type enzyme: S0.5 for D-erythrose-4-phophate equal to 7.1 X 10-4 M; S0.5 for phosphoenolpyruvate equal to 1.4 X 10-4 M. Inhibition by tyrosine was mixed with respect to binding of both D-erythrose-4-phosphate and phosphoenolpyruvate. In addition, tyrosine promoted cooperative interactions in the binding of phosphoenolpyruvate. For the altered enzyme the following apparent constants were obtained: S0.5 for D-erythrose-4-phosphate equal to 7.1 X 10-4 M; S0.5 for phosphoenolpyruvate equal to 2.9 X 10-4 M. Inhibition by tyrosine was mixed with respect to D-erythrose-4-phosphate and competitive with respect to phosphoenolpyruvate. Tyrosine did not promote cooperative effects in the binding of phosphoenolpyruvate to the altered enzyme.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., GARRY B., HERZENBERG L. A. The genetic control of the enzymes of histidine biosynthesis in Salmonella typhimurium. J Gen Microbiol. 1960 Apr;22:369–378. doi: 10.1099/00221287-22-2-369. [DOI] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISCHE Z., DISCHE M. R. Methods for the qualitative and quantitative determinations of tetroses by two new specific color reactions. Biochim Biophys Acta. 1958 Jan;27(1):184–188. doi: 10.1016/0006-3002(58)90307-x. [DOI] [PubMed] [Google Scholar]
- Di Jeso F. Ammonium sulfate concentration conversion nomograph for 0 degrees. J Biol Chem. 1968 Apr 25;243(8):2022–2023. [PubMed] [Google Scholar]
- Holtzclaw W. D., Chapman L. F., Gowans C. S. Some regulatory properties of phospho-2-keto-3-deoxyheptonate aldolase from the blue-green alga Anacystis nidulans. Biochim Biophys Acta. 1972 May 12;268(2):562–572. doi: 10.1016/0005-2744(72)90353-1. [DOI] [PubMed] [Google Scholar]
- MOYED H. S. False feedback inhibition: inhibition of tryptophan biosynthesis by 5-methyltryptophan. J Biol Chem. 1960 Apr;235:1098–1102. [PubMed] [Google Scholar]
- MOYED H. S. Interference with the feed-back control of histidine biosynthesis. J Biol Chem. 1961 Aug;236:2261–2267. [PubMed] [Google Scholar]
- SHEPPARD D. E. MUTANTS OF SALMONELLA TYPHIMURIUM RESISTANT TO FEEDBACK INHIBITION BY L-HISTIDINE. Genetics. 1964 Oct;50:611–623. doi: 10.1093/genetics/50.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]


