Abstract
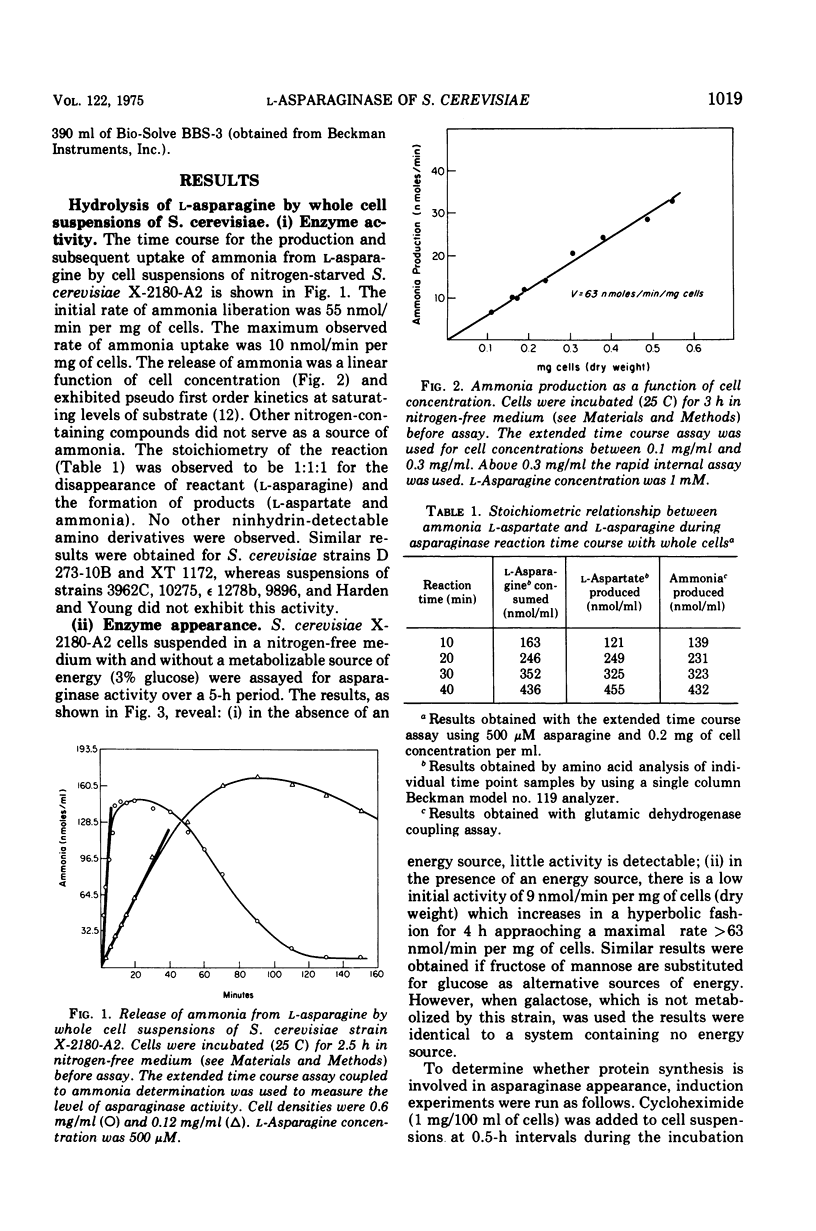
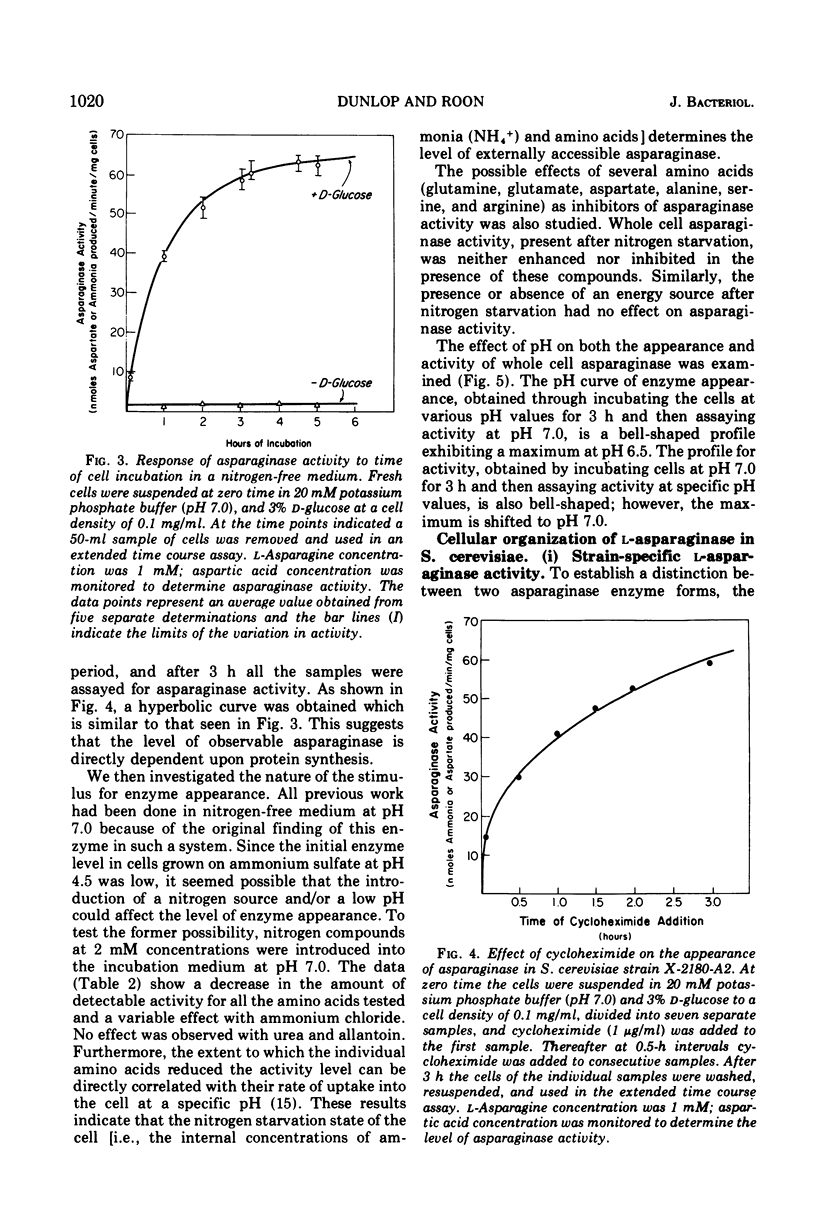
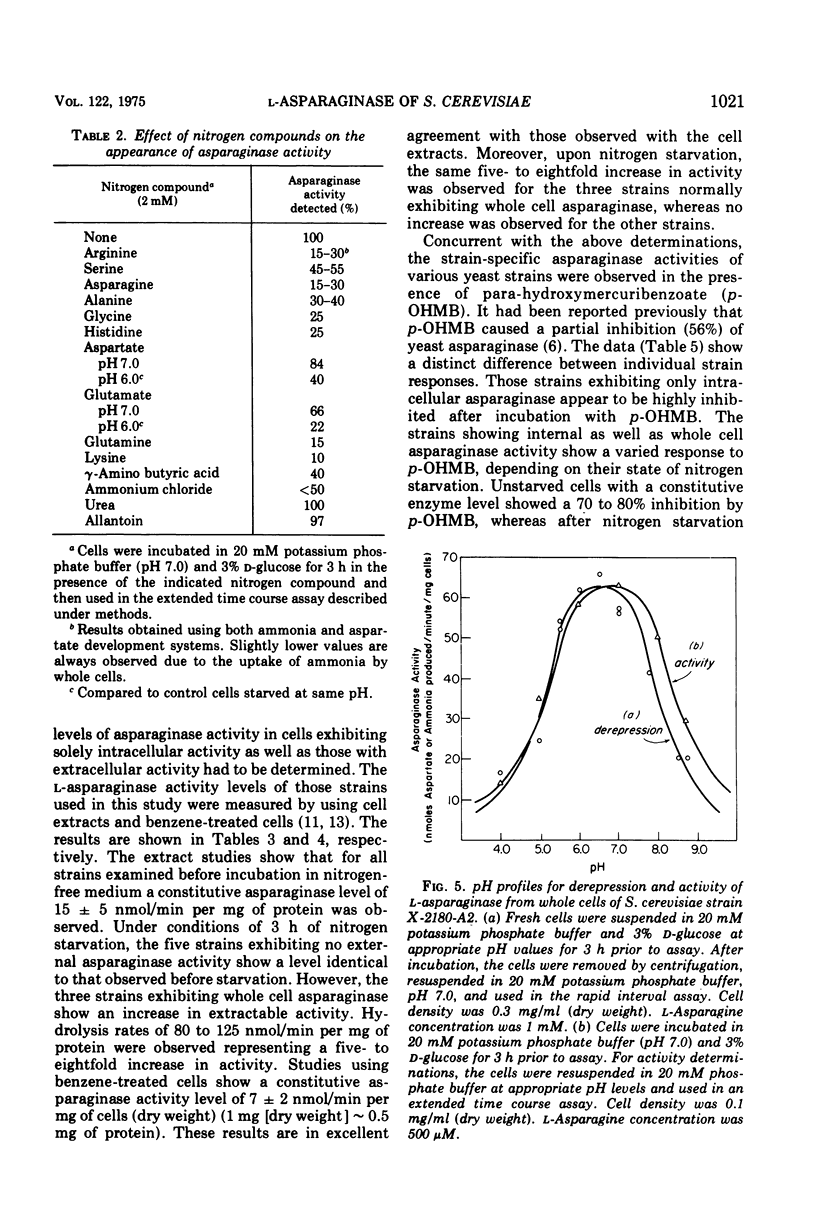
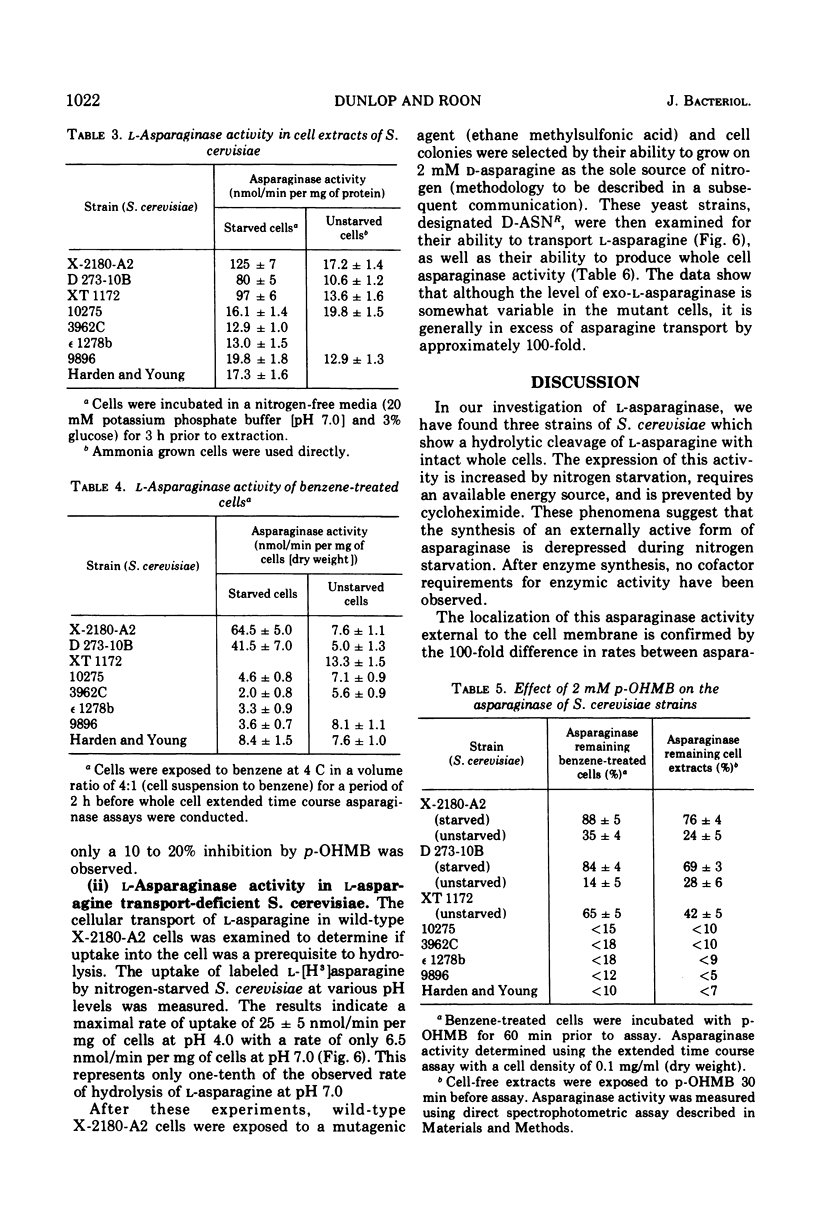
During recent studies conducted with suspensions of three strains of Saccharomyces cerevisiae, it was observed that ammonia was rapidly liberated when L-asparagine was added to the medium. Subsequent investigation has revealed that these strains of S. cerevisiae have an externally active asparaginase as well as an internally active one. The appearance of the external asparaginase is stimulated by nitrogen starvation, requires an available energy source, and is prevented by cycloheximide. The internal enzyme appears to be constitutive. The external activity is relatively insensitive to para-hydroxymercuribenzoate inhibition, whereas the internal activity is highly inhibited by this compound.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdumalikov A. Kh, Eremenko V. V. Gliutaminaza i izogliutaminaza Saccharomyces cerevisciae Meyen KMY 381. Biokhimiia. 1967 Mar-Apr;32(2):363–367. [PubMed] [Google Scholar]
- Broome J. D. Antilymphoma activity of L-asparaginase in vivo: clearance rates of enzyme preparations from guinea pig serum and yeast in relation to their effect on tumor growth. J Natl Cancer Inst. 1965 Dec;35(6):967–974. [PubMed] [Google Scholar]
- Cedar H., Schwartz J. H. Localization of the two-L-asparaginases in anaerobically grown Escherichia coli. J Biol Chem. 1967 Aug 25;242(16):3753–3755. [PubMed] [Google Scholar]
- Farkas V., Svoboda A., Bauer S. Secretion of cell-wall glycoproteins by yeast protoplasts. Effect of 2-deoxy-D-glucose and cycloheximide. Biochem J. 1970 Aug;118(5):755–758. doi: 10.1042/bj1180755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. E. A fine-structure map of the yeast l-asparaginase gene. Mol Gen Genet. 1973;121(1):9–14. doi: 10.1007/BF00353689. [DOI] [PubMed] [Google Scholar]
- Jones G. E., Mortimer R. K. Biochemical properties of yeast L-asparaginase. Biochem Genet. 1973 Jun;9(2):131–146. doi: 10.1007/BF00487443. [DOI] [PubMed] [Google Scholar]
- Jones G. E., Mortimer R. K. L-asparaginase-deficient mutants of yeast. Science. 1970 Jan 9;167(3915):181–182. doi: 10.1126/science.167.3915.181. [DOI] [PubMed] [Google Scholar]
- Magee P. T., Robichon-Szulmajster H. The regulation of isoleucine-valine biosynthesis in Saccharomyces cerevisiae. 3. Properties and regulation of the activity of acetohydroxyacid synthetase. Eur J Biochem. 1968 Feb;3(4):507–511. doi: 10.1111/j.1432-1033.1967.tb19560.x. [DOI] [PubMed] [Google Scholar]
- Selwyn M. J. A simple test for inactivation of an enzyme during assay. Biochim Biophys Acta. 1965 Jul 29;105(1):193–195. doi: 10.1016/s0926-6593(65)80190-4. [DOI] [PubMed] [Google Scholar]
- Serrano R., Gancedo J. M., Gancedo C. Assay of yeast enzymes in situ. A potential tool in regulation studies. Eur J Biochem. 1973 May 2;34(3):479–482. doi: 10.1111/j.1432-1033.1973.tb02783.x. [DOI] [PubMed] [Google Scholar]
- Surdin Y., Sly W., Sire J., Bordes A. M., Robichon-Szulmajster H. Propriétés et contrôle génétique du système d'accumulation des acides aminés chez Saccharomyces cerevisiae. Biochim Biophys Acta. 1965 Oct 18;107(3):546–566. [PubMed] [Google Scholar]
- Wriston J. C., Jr, Yellin T. O. L-asparaginase: a review. Adv Enzymol Relat Areas Mol Biol. 1973;39:185–248. doi: 10.1002/9780470122846.ch3. [DOI] [PubMed] [Google Scholar]



