Abstract
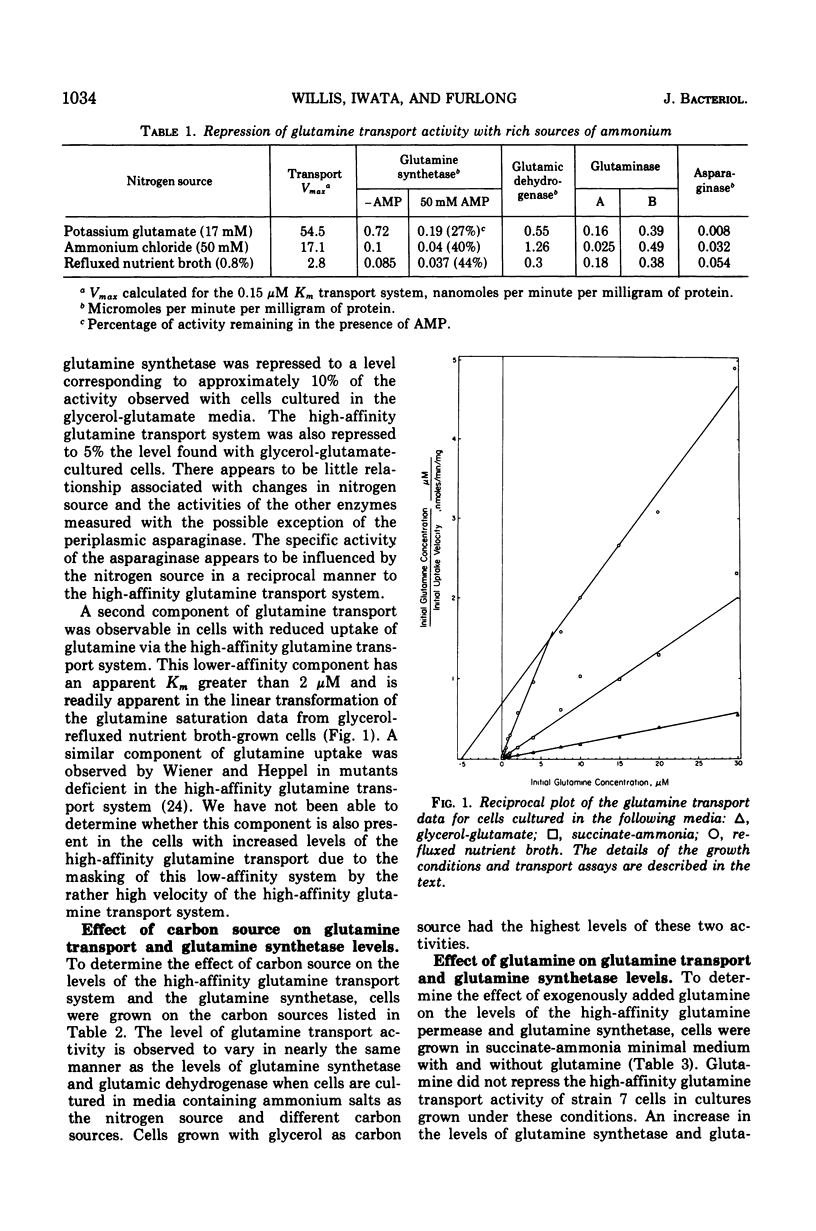
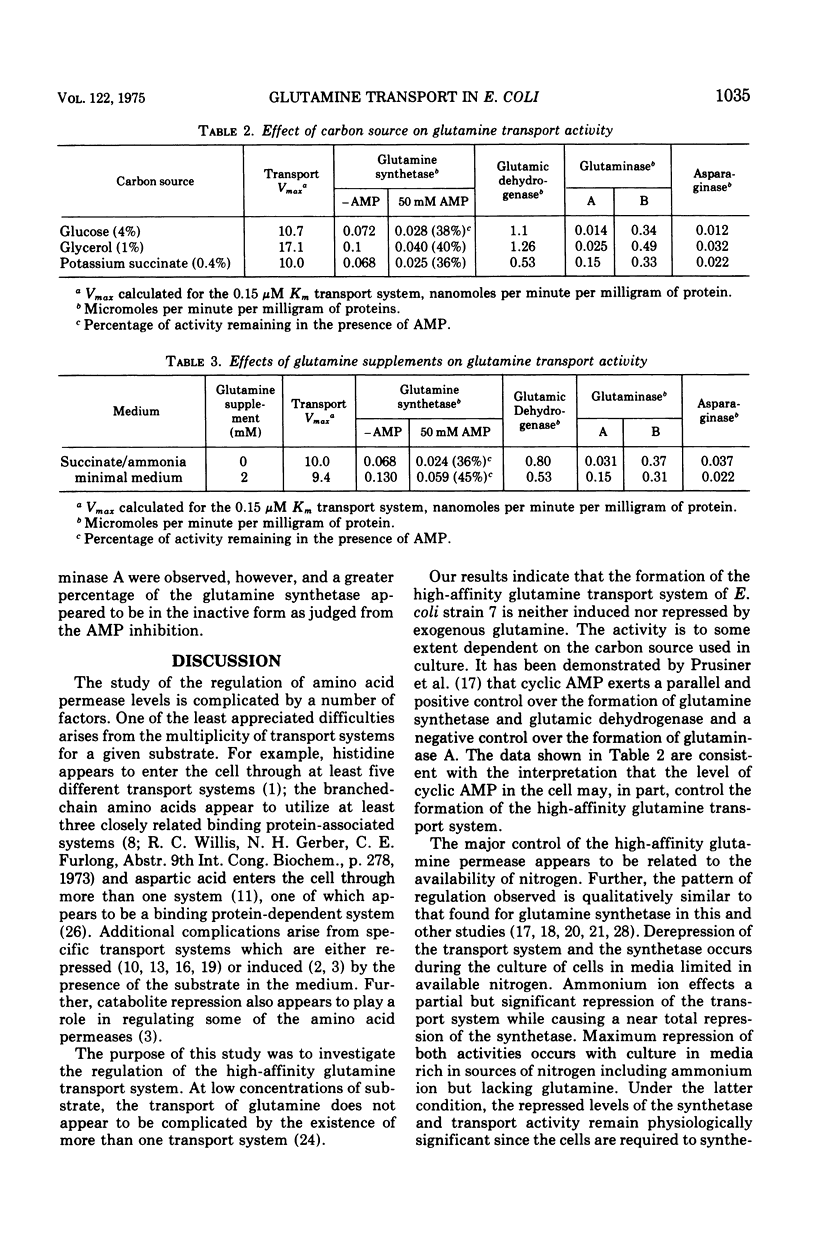
The formation of the high-affinity (Km equal to 0.2 muM) L-glutamine transport system of Escherichia coli strain 7 (Lin) appears to be subject to the same major control as the glutamine synthetase (EC 6.3.1.2) of this gram-negative organism. Culture of cells under nitrogen-limited conditions provides maximum derepression of both the glutamine synthetase and the glutamine transport system. Nutritional conditions providing a rich supply of ammonium salts or available sources of nitrogen, i.e., conditions which repress the formation of glutamine synthetase, provide three- and 20-fold repression, respectively, of the glutamine transport system. Culture of cells with glutamine supplements of 2 mM does not increase the repression of high-affinity glutamine transport system beyond the level observed in the absence of glutamine. A second kinetically distinct low-affinity component of glutamine. A second kinetically distinct low-affinity component of glutamine uptake is observed in cells cultured with a glutamine-depleted nutrient broth. This second component is associated with the appearance of glutaminase A (EC 3.5.1.2) and asparaginase I (EC 3.5.1.1), a periplasmic enzyme. Parallel changes were observed in the levels of the high-affinity glutamine transport system and the glutamine synthetase when cells were cultured with the carbon sources: glucose, glycerol, or succinate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Lever J. E. The histidine-binding protein J is a component of histidine transport. Identification of its structural gene, hisJ. J Biol Chem. 1972 Jul 10;247(13):4309–4316. [PubMed] [Google Scholar]
- BURROUS S. E., DEMOSS R. D. STUDIES ON TRYPTOPHAN PERMEASE IN ESCHERICHIA COLI. Biochim Biophys Acta. 1963 Aug 6;73:623–637. doi: 10.1016/0006-3002(63)90332-9. [DOI] [PubMed] [Google Scholar]
- Campbell H. A., Mashburn L. T., Boyse E. A., Old L. J. Two L-asparaginases from Escherichia coli B. Their separation, purification, and antitumor activity. Biochemistry. 1967 Mar;6(3):721–730. doi: 10.1021/bi00855a011. [DOI] [PubMed] [Google Scholar]
- DOWD J. E., RIGGS D. S. A COMPARISON OF ESTIMATES OF MICHAELIS-MENTEN KINETIC CONSTANTS FROM VARIOUS LINEAR TRANSFORMATIONS. J Biol Chem. 1965 Feb;240:863–869. [PubMed] [Google Scholar]
- Furlong C. E., Cirakoglu C., Willis R. C., Santy P. A. A simple preparative polyacrylamide disc gel electrophoresis apparatus: purification of three branched-chain amino acid binding proteins from Escherichia coli. Anal Biochem. 1973 Jan;51(1):297–311. doi: 10.1016/0003-2697(73)90478-8. [DOI] [PubMed] [Google Scholar]
- INUI Y., AKEDO H. AMINO ACID UPTAKE BY ESCHERICHIA COLI GROWN IN PRESENCE OF AMINO ACIDS. EVIDENCE FOR REPRESSIBILITY OF AMINO ACID UPTAKE. Biochim Biophys Acta. 1965 Jan 25;94:143–152. doi: 10.1016/0926-6585(65)90018-x. [DOI] [PubMed] [Google Scholar]
- KOCH J. P., HAYASHI S., LIN E. C. THE CONTROL OF DISSIMILATION OF GLYCEROL AND L-ALPHA-GLYCEROPHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1964 Sep;239:3106–3108. [PubMed] [Google Scholar]
- Kay W. W. Two aspartate transport systems in Escherichia coli. J Biol Chem. 1971 Dec 10;246(23):7373–7382. [PubMed] [Google Scholar]
- Lankford C. E., Snell E. E. Glutamine as a Growth Factor for Certain Strains of Neisseria Gonorrhoeae. J Bacteriol. 1943 Apr;45(4):410–411. doi: 10.1128/jb.45.4.410-411.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penrose W. R., Nichoalds G. E., Piperno J. R., Oxender D. L. Purification and properties of a leucine-binding protein from Escherichia coli. J Biol Chem. 1968 Nov 25;243(22):5921–5928. [PubMed] [Google Scholar]
- Prusiner S., Miller R. E., Valentine R. C. Adenosine 3':5'-cyclic monophosphate control of the enzymes of glutamine metabolism in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2922–2926. doi: 10.1073/pnas.69.10.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen B. P. Basic amino acid transport in Escherichia coli. J Biol Chem. 1971 Jun 10;246(11):3653–3662. [PubMed] [Google Scholar]
- Schutt H., Holzer H. Biological function of the ammonia-induced inactivation of glutamine synthetase in Escherichia coli. Eur J Biochem. 1972 Mar 15;26(1):68–72. doi: 10.1111/j.1432-1033.1972.tb01740.x. [DOI] [PubMed] [Google Scholar]
- Varricchio F., Holzer H. Independent genetic regulation of glutamine synthetase and its inactivating (adenylating) enzyme in E. coli. FEBS Lett. 1969 Jun;3(4):263–264. doi: 10.1016/0014-5793(69)80153-5. [DOI] [PubMed] [Google Scholar]
- Vogel H. J. REPRESSION OF AN ACETYLORNITHINE PERMEATION SYSTEM. Proc Natl Acad Sci U S A. 1960 Apr;46(4):488–494. doi: 10.1073/pnas.46.4.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner J. H., Furlong C. E., Heppel L. A. A binding protein for L-glutamine and its relation to active transport in E. coli. Arch Biochem Biophys. 1971 Feb;142(2):715–717. doi: 10.1016/0003-9861(71)90538-8. [DOI] [PubMed] [Google Scholar]
- Willis R. C., Furlong C. E. Purification and properties of a periplasmic glutamate-aspartate binding protein from Escherichia coli K12 strain W3092. J Biol Chem. 1975 Apr 10;250(7):2574–2580. [PubMed] [Google Scholar]
- Woolfolk C. A., Shapiro B., Stadtman E. R. Regulation of glutamine synthetase. I. Purification and properties of glutamine synthetase from Escherichia coli. Arch Biochem Biophys. 1966 Sep 26;116(1):177–192. doi: 10.1016/0003-9861(66)90026-9. [DOI] [PubMed] [Google Scholar]
- Wu H. C., Boos W., Kalckar H. M. Role of the galactose transport system in the retention of intracellular galactose in Escherichia coli. J Mol Biol. 1969 Apr 14;41(1):109–120. doi: 10.1016/0022-2836(69)90129-6. [DOI] [PubMed] [Google Scholar]



