Abstract
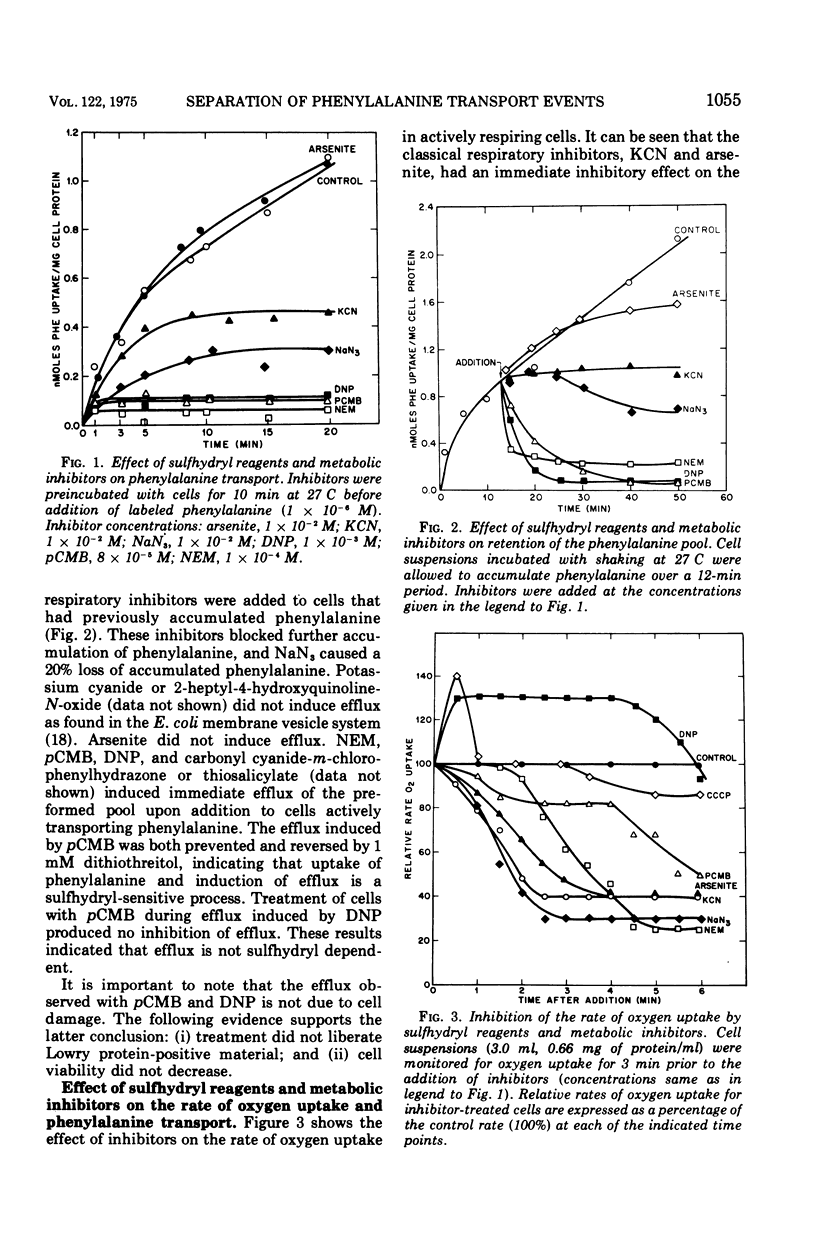
Phenylalanine transport in Yersinia pestis TJW was differentially inhibited by sulfhydryl blocking reagents, uncoupling agents, and respiratory inhibitors. Kinetic studies with potassium cyanide and sodium azide showed that these compounds have no immediate effect on the initial rate of phenylalanine transport, but have an immediate and severe inhibitory effect on the rate of oxygen uptake. Identical studies with p-chloromercuribenzoate (pCMB) and 2,4-dinitrophenol (DNP) showed that these compounds have an instantaneous and total inhibitory effect on phenylalanine transport. DNP stimulated oxygen uptake, and pCMB caused only a sluggish inhibiton of oxygen uptake. pCMB acted as a competitive inhibitor of phenylalanine transport, whereas DNP inhibitied noncompetitively. Arrenius plots of the initial rate of phenylalanine transport in pCMB- and DNP-treated cells showed that DNP alters the transition temperature of the phenylalanine transport system from 17 C for control cells to 12 C. DNP did not inhibit transport when cells were treated at temperatures of 2 to 10 C. PCMB did not alter the normal transition temperature and inhibited phenylalanine transport over a 2 to 30 C temperature range. Efflux induced by both pCMB and DNP were blocked by placing cells at low temperatures (2 to 20 C). Inhibition of adenosine 5'-triphosphate synthesis by DNP did not show any temperature sensitivity as did phenylalanine transport. These data indicate that: (i) respiration is not obligatory for active transport of phenylalanine in Y. pestis TJW; and (ii) pCMB inhibits transport activity by reacting with the sulfhydryl group(s) at the carrier binding site. The data show that the uncoupler, DNP, selectively alters a temperature-dependent property of phenylalanine transport, that is not related to uncoupling activity of DNP , and probably involves membrane lipid alterations.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakker E. P., van den Heuvel E. J., Wiechmann A. H., van Dam K. A comparison between the effectiveness of uncouplers of oxidative phosphorylation in mitochondria and in different artificial membrane systems. Biochim Biophys Acta. 1973 Jan 18;292(1):78–87. doi: 10.1016/0005-2728(73)90252-1. [DOI] [PubMed] [Google Scholar]
- Berger E. A. Different mechanisms of energy coupling for the active transport of proline and glutamine in Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1514–1518. doi: 10.1073/pnas.70.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boos W. Bacterial transport. Annu Rev Biochem. 1974;43(0):123–146. doi: 10.1146/annurev.bi.43.070174.001011. [DOI] [PubMed] [Google Scholar]
- Cunarro J., Weiner M. W. Quantitative correlation between the proton-carrying and respiratory-stimulating properties of uncoupling agents using rat liver mitochondria. Nature. 1973 Sep 7;245(5419):36–37. doi: 10.1038/245036a0. [DOI] [PubMed] [Google Scholar]
- Engelman D. M. Lipid bilayer structure in the membrane of Mycoplasma laidlawii. J Mol Biol. 1971 May 28;58(1):153–165. doi: 10.1016/0022-2836(71)90238-5. [DOI] [PubMed] [Google Scholar]
- Engelman D. M. X-ray diffraction studies of phase transitions in the membrane of Mycoplasma laidlawii. J Mol Biol. 1970 Jan 14;47(1):115–117. doi: 10.1016/0022-2836(70)90407-9. [DOI] [PubMed] [Google Scholar]
- Esfahani M., Limbrick A. R., Knutton S., Oka T., Wakil S. J. The molecular organization of lipids in the membrane of Escherichia coli: phase transitions. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3180–3184. doi: 10.1073/pnas.68.12.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster M., McLaughlin S. Complexes between uncouplers of oxidative phosphorylation. J Membr Biol. 1974;17(2):155–180. doi: 10.1007/BF01870177. [DOI] [PubMed] [Google Scholar]
- Fox C. F., Kennedy E. P. Specific labeling and partial purification of the M protein, a component of the beta-galactoside transport system of Escherichia coli. Proc Natl Acad Sci U S A. 1965 Sep;54(3):891–899. doi: 10.1073/pnas.54.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern Y. S., Barash H., Druck K. Glutamate transport in Escherichia coli K-12: nonidentity of carriers mediating entry and exit. J Bacteriol. 1973 Jan;113(1):51–57. doi: 10.1128/jb.113.1.51-57.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Inhibition of membrane transport in Streptococcus faecalis by uncouplers of oxidative phosphorylation and its relationship to proton conduction. J Bacteriol. 1968 Dec;96(6):2025–2034. doi: 10.1128/jb.96.6.2025-2034.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R. A., Penniston J. T., Asai J., Green D. E. The conformational basis of energy conservation in membrane systems. II. Correlation between conformational change and functional states. Proc Natl Acad Sci U S A. 1968 Mar;59(3):830–837. doi: 10.1073/pnas.59.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden J. T., Utech N. M., Hegeman G. D., Kenyon C. N. Heterogeneous changes in amino acid transport activity in E. coli lipid overproducing mutants. Biochem Biophys Res Commun. 1973 Jan 23;50(2):266–272. doi: 10.1016/0006-291x(73)90835-8. [DOI] [PubMed] [Google Scholar]
- Hong J. S., Kaback H. R. Mutants of Salmonella typhimurium and Escherichia coli pleiotropically defective in active transport. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3336–3340. doi: 10.1073/pnas.69.11.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R., Barnes E. M., Jr Mechanisms of active transport in isolated membrane vesicles. II. The mechanism of energy coupling between D-lactic dehydrogenase and beta-galactoside transport in membrane preparations from Escherichia coli. J Biol Chem. 1971 Sep 10;246(17):5523–5531. [PubMed] [Google Scholar]
- Kaback H. R., Reeves J. P., Short S. A., Lombardi F. J. Mechanisms of active transport in isolated bacterial membrane vesicles. 18. The mechanism of action of carbonylcyanide m-chlorophenylhydrazone. Arch Biochem Biophys. 1974 Jan;160(1):215–222. doi: 10.1016/s0003-9861(74)80028-7. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Transport across isolated bacterial cytoplasmic membranes. Biochim Biophys Acta. 1972 Aug 4;265(3):367–416. doi: 10.1016/0304-4157(72)90014-7. [DOI] [PubMed] [Google Scholar]
- Kerwar G. K., Gordon A. S., Kaback H. R. Mechanisms of active transport in isolated membrane vesicles. IV. Galactose transport by isolated membrane vesicles from Escherichia coli. J Biol Chem. 1972 Jan 10;247(1):291–297. [PubMed] [Google Scholar]
- Klein W. L., Boyer P. D. Energization of active transport by Escherichia coli. J Biol Chem. 1972 Nov 25;247(22):7257–7265. [PubMed] [Google Scholar]
- Mavis R. D., Vagelos P. R. The effect of phospholipid fatty acid composition in membranous enzymes in Escherichia coli. J Biol Chem. 1972 Feb 10;247(3):652–659. [PubMed] [Google Scholar]
- OYAMA V. I., EAGLE H. Measurement of cell growth in tissue culture with a phenol reagent (folin-ciocalteau). Proc Soc Exp Biol Med. 1956 Feb;91(2):305–307. doi: 10.3181/00379727-91-22245. [DOI] [PubMed] [Google Scholar]
- Parnes J. R., Boos W. Energy coupling of the -methylgalactoside transport system of Escherichia coli. J Biol Chem. 1973 Jun 25;248(12):4429–4435. [PubMed] [Google Scholar]
- Parnes J. R., Boos W. Unidirectional transport activity mediated by the galactose-binding protein of Escherichia coli. J Biol Chem. 1973 Jun 25;248(12):4436–4445. [PubMed] [Google Scholar]
- Pavlasova E., Harold F. M. Energy coupling in the transport of beta-galactosides by Escherichia coli: effect of proton conductors. J Bacteriol. 1969 Apr;98(1):198–204. doi: 10.1128/jb.98.1.198-204.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. B., Montie T. C. Aromatic amino acid transport in Yersinia pestis. J Bacteriol. 1975 Jun;122(3):1045–1052. doi: 10.1128/jb.122.3.1045-1052.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P. E., Williams S. G. Use of the liquid scintillation spectrometer for determining adenosine triphosphate by the luciferase enzyme. Anal Biochem. 1969 Jun;29(3):381–392. doi: 10.1016/0003-2697(69)90323-6. [DOI] [PubMed] [Google Scholar]
- Ting H. P., Wilson D. F., Chance B. Effects of uncouplers of oxidative phosphorylation on the specific conductance of bimolecular lipid membranes. Arch Biochem Biophys. 1970 Nov;141(1):141–146. doi: 10.1016/0003-9861(70)90116-5. [DOI] [PubMed] [Google Scholar]
- Tsukagoshi N., Fox C. F. Abortive assembly of the lactose transport system in Escherichia coli. Biochemistry. 1973 Jul 17;12(15):2816–2822. doi: 10.1021/bi00739a007. [DOI] [PubMed] [Google Scholar]
- Verma S. P., Schneider H., Smith I. C. Organizational changes in phospholipid multibilayers induced by uncouplers of oxidative phosphorylation: a spin label study. Arch Biochem Biophys. 1973 Jan;154(1):400–406. doi: 10.1016/0003-9861(73)90072-6. [DOI] [PubMed] [Google Scholar]
- West I. C., Mitchell P. Stoicheiometry of lactose-H+ symport across the plasma membrane of Escherichia coli. Biochem J. 1973 Mar;132(3):587–592. doi: 10.1042/bj1320587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West I. C., Wilson T. H. Galactoside transport dissociated from proton movement in mutants of Escherichia coli. Biochem Biophys Res Commun. 1973 Jan 23;50(2):551–558. doi: 10.1016/0006-291x(73)90875-9. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Ting H. P., Koppelman M. S. Mechanism of action of uncouplers of oxidative phosphorylation. Biochemistry. 1971 Jul 20;10(15):2897–2902. doi: 10.1021/bi00791a016. [DOI] [PubMed] [Google Scholar]
- Wilson G., Fox C. F. Biogenesis of microbial transport systems: evidnce for coupled incorporation of newly synthesized lipids and proteins into membrane. J Mol Biol. 1971 Jan 14;55(1):49–60. doi: 10.1016/0022-2836(71)90280-4. [DOI] [PubMed] [Google Scholar]
- Young J. H., Blondin G. A., Vanderkooi G., Green D. E. Conformational model of active transport. Proc Natl Acad Sci U S A. 1970 Oct;67(2):550–559. doi: 10.1073/pnas.67.2.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Thienen G., Postma P. W. Coupling between energy conservation and active transport of serine in Escherichia coli. Biochim Biophys Acta. 1973 Oct 25;323(3):429–440. doi: 10.1016/0005-2736(73)90188-0. [DOI] [PubMed] [Google Scholar]



