Abstract
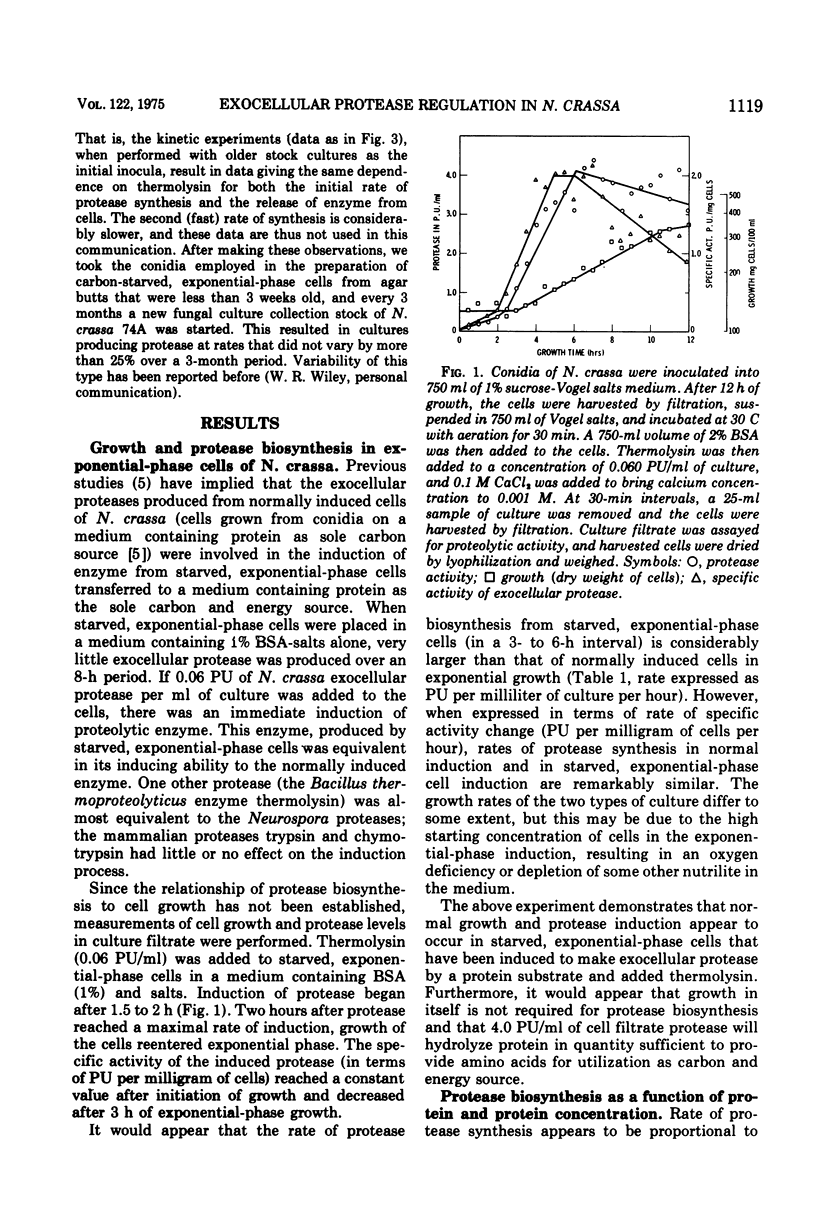
To induce exocellular proteolytic enzyme from carbon-starved exponential-phase cells of Neurospora crassa, both a protein substrate and an activating protease of certain specific properties must be present at the same time. The cells must be capable of protein synthesis, since cycloheximide inhibits the process, but cell growth, as determined by increase in cell mass, does not appear to be required. Both soluble (bovine serum albumin, myoglobin) and insoluble protein substrates (collagen, corn zein) will affect protease induction, although certain soluble, globular proteins (egg white globulin, bovine gamma globulin) will not. In most cases, rates of protease induction are proportional to protein concentration, regardless of the nature of the inducing protein. All activating proteases capable of affecting induction in a manner similar to that of N. crassa exocellular protease were of bacterial origin and were exoproteases. Mammalian proteases and peptidases had little or no effect on the induction process.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen B. L. Regulation of intracellular and extracellular neutral and alkaline proteases in Aspergillus nidulans. J Gen Microbiol. 1973 Dec;79(2):311–320. doi: 10.1099/00221287-79-2-311. [DOI] [PubMed] [Google Scholar]
- Cohen B. L. The neutral and alkaline proteases of Aspergillus nidulans. J Gen Microbiol. 1973 Aug;77(2):521–528. doi: 10.1099/00221287-77-2-521. [DOI] [PubMed] [Google Scholar]
- Drucker H. Regulation of exocellular proteases in Neurospora crassa: induction and repression of enzyme synthesis. J Bacteriol. 1972 Jun;110(3):1041–1049. doi: 10.1128/jb.110.3.1041-1049.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker H. Regulation of exocellular proteases in Neurospora crassa: role of Neurospora proteases in induction. J Bacteriol. 1973 Nov;116(2):593–599. doi: 10.1128/jb.116.2.593-599.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLK J. E., SCHIRMER E. W. THE PORCINE PANCREATIC CARBOXYPEPTIDASE A SYSTEM. I. THREE FORMS OF THE ACTIVE ENZYME. J Biol Chem. 1963 Dec;238:3884–3894. [PubMed] [Google Scholar]
- Hanson M. A., Marzluf G. A. Regulation of a sulfur-controlled protease in Neurospora crassa. J Bacteriol. 1973 Nov;116(2):785–789. doi: 10.1128/jb.116.2.785-789.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDL I., MACLENNAN J. D., HOWES E. L. Isolation and characterization of proteinase and collagenase from Cl. histolyticum. J Clin Invest. 1953 Dec;32(12):1323–1329. doi: 10.1172/JCI102861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCDONALD C. E., CHEN L. L. THE LOWRY MODIFICATION OF THE FOLIN REAGENT FOR DETERMINATION OF PROTEINASE ACTIVITY. Anal Biochem. 1965 Jan;10:175–177. doi: 10.1016/0003-2697(65)90255-1. [DOI] [PubMed] [Google Scholar]
- Morihara K., Oka T., Tsuzuki H. Comparative study of various serine proteinases from microorganisms: specificity with oligopeptides. Arch Biochem Biophys. 1971 Sep;146(1):297–305. doi: 10.1016/s0003-9861(71)80067-x. [DOI] [PubMed] [Google Scholar]
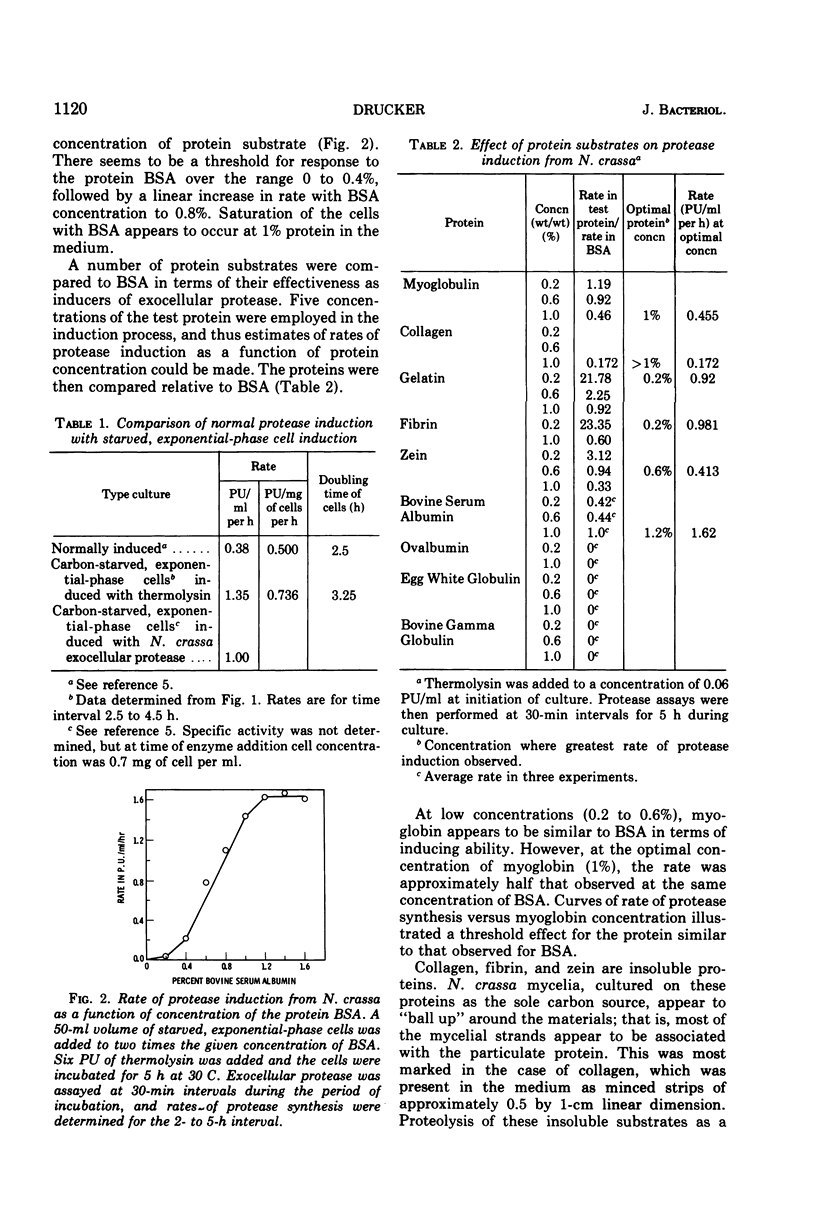
- Morihara K., Tsuzuki H. Comparative study of various neutral proteinases from microorganisms: specificity with oligopeptides. Arch Biochem Biophys. 1971 Sep;146(1):291–296. doi: 10.1016/s0003-9861(71)80066-8. [DOI] [PubMed] [Google Scholar]
- NAUGHTON M. A., SANGER F. Purification and specificity of pancreatic elastase. Biochem J. 1961 Jan;78:156–163. doi: 10.1042/bj0780156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleniacz W. S., Pisano M. A. Proteinase production by a species of Cephalosporium. Appl Microbiol. 1968 Jan;16(1):90–96. doi: 10.1128/am.16.1.90-96.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
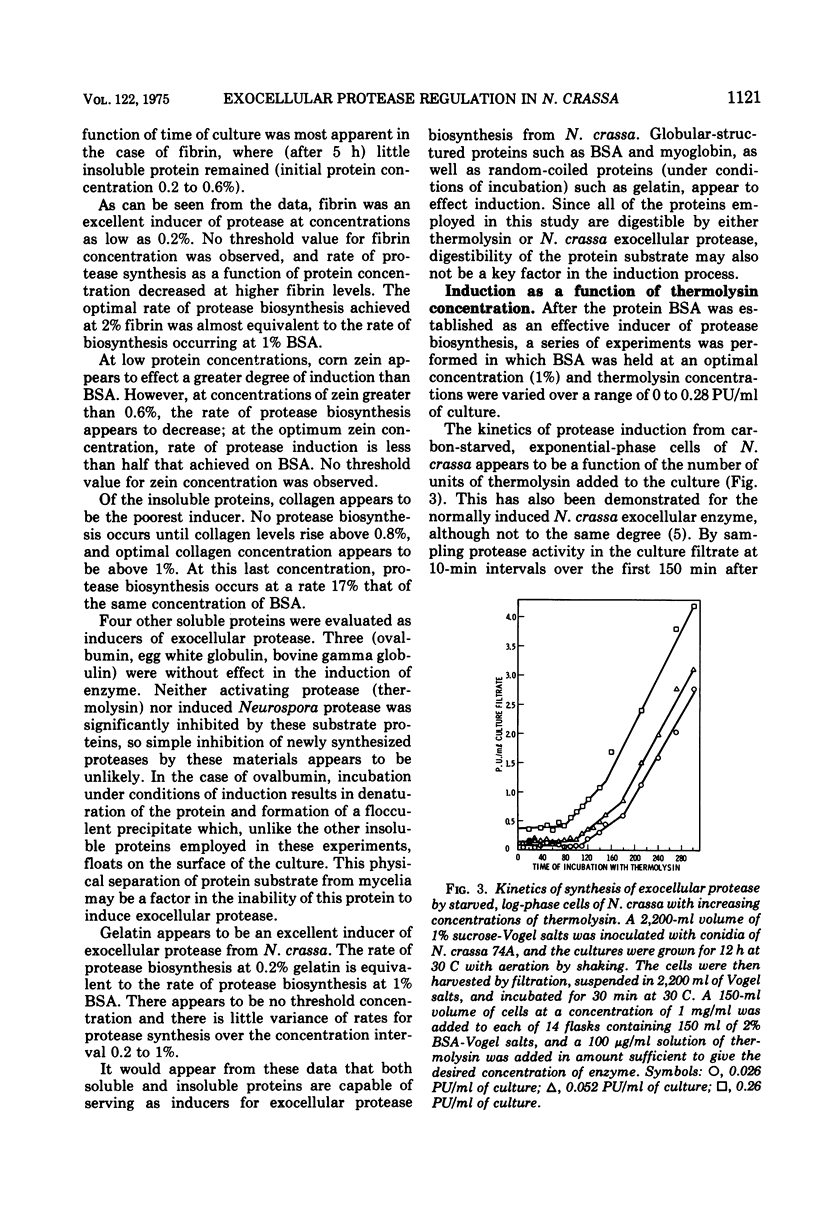
- Roncari G., Zuber H. Thermophilic aminopeptidases from Bacillus stearothermophilus. I. Isolation, specificity, and general properties of the thermostable aminopeptidase I. Int J Protein Res. 1969;1(1):45–61. doi: 10.1111/j.1399-3011.1969.tb01625.x. [DOI] [PubMed] [Google Scholar]
- SANGER F., THOMPSON E. O. P. The amino-acid sequence in the glycyl chain of insulin. II. The investigation of peptides from enzymic hydrolysates. Biochem J. 1953 Feb;53(3):366–374. doi: 10.1042/bj0530366. [DOI] [PMC free article] [PubMed] [Google Scholar]
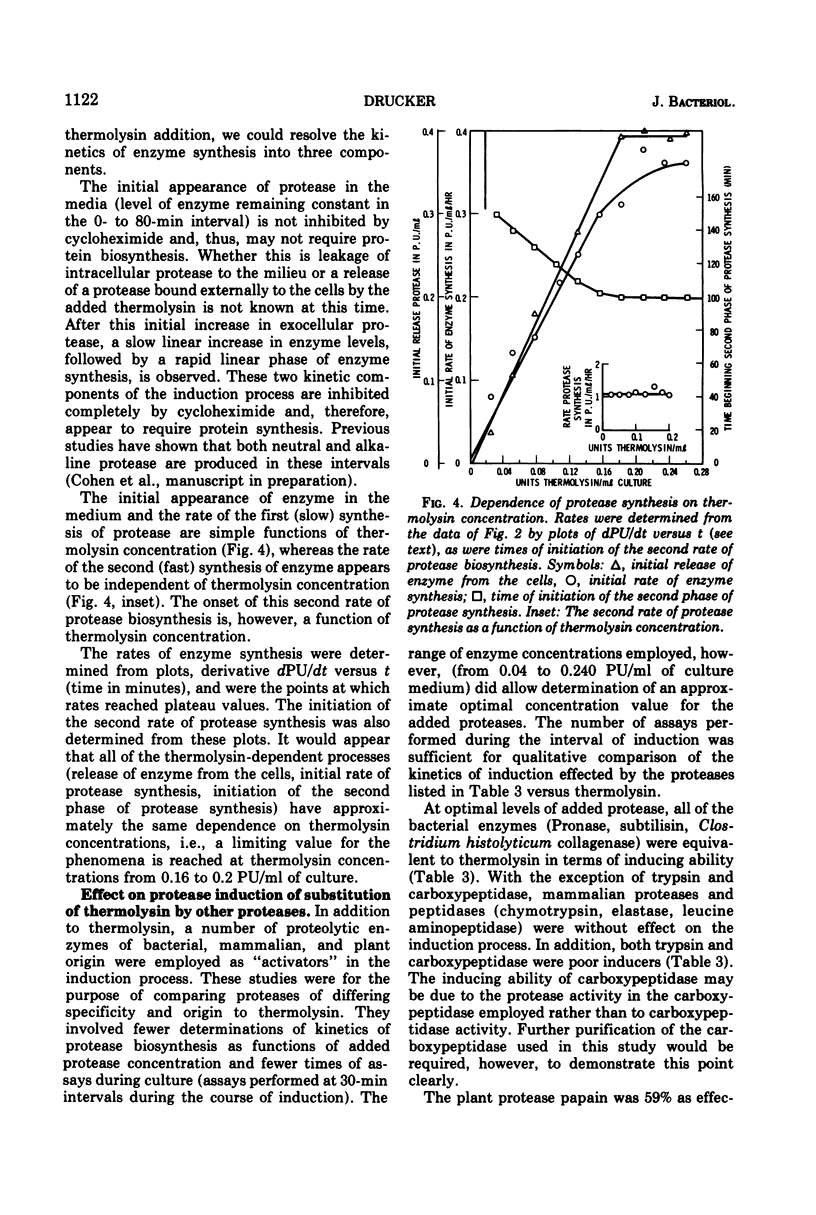
- Schneider R. P., Wiley W. R. Transcription and degradation of messenger ribonucleic acid for a glucose transport system in Neurospora. J Biol Chem. 1971 Aug 10;246(15):4784–4789. [PubMed] [Google Scholar]
- Shirato S., Nagatsu C. Fermentation studies with Streptomyces griseus. I. Carbohydrate sources for the production of protease and streptomycin. Appl Microbiol. 1965 Sep;13(5):669–672. doi: 10.1128/am.13.5.669-672.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somkuti G. A., Babel F. J. Conditions influencing the synthesis of acid protease by Mucor pusillus Lindt. Appl Microbiol. 1967 Nov;15(6):1309–1312. doi: 10.1128/am.15.6.1309-1312.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. L. Release of proteinase from mycelium of Mucor hiemalis. J Bacteriol. 1967 Jun;93(6):1794–1799. doi: 10.1128/jb.93.6.1794-1799.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]



