Abstract
Sequence-selective transcription by bacterial RNA polymerase (RNAP) requires σ factor that participates in both promoter recognition and DNA melting. RNAP lacking σ (core enzyme) will initiate RNA synthesis from duplex ends, nicks, gaps, and single-stranded regions. We have used DNA templates containing short regions of heteroduplex (bubbles) to compare initiation in the presence and absence of various σ factors. Using bubble templates containing the σD-dependent flagellin promoter, with or without its associated upstream promoter (UP) element, we demonstrate that UP element stimulation occurs efficiently even in the absence of σ. This supports a model in which the UP element acts primarily through the α subunit of core enzyme to increase the initial association of RNAP with the promoter. Core and holoenzyme do differ substantially in the template positions chosen for initiation: σD restricts initiation to sites 8–9 nucleotides downstream of the conserved −10 element. Remarkably, σA also has a dramatic effect on start-site selection even though the σA holoenzyme is inactive on the corresponding homoduplexes. The start sites chosen by the σA holoenzyme are located 8 nucleotides downstream of sequences on the nontemplate strand that resemble the conserved −10 hexamer recognized by σA. Thus, σA appears to recognize the −10 region even in a single-stranded state. We propose that in addition to its described roles in promoter recognition and start-site melting, σ also localizes the transcription start site.
Keywords: transcription, promoter, flagellin, Bacillus subtilis
Bacterial RNA polymerase (RNAP) is a multimeric enzyme with minimal subunit composition ββ′α2σ. Core enzyme (ββ′α2 or E) contains the polymerase catalytic function but must associate with σ, forming a holoenzyme (Eσ), before promoter recognition can occur (for review see ref. 1). Transcription initiation involves at least three distinct intermediates: an initial closed complex, a strand-separated open complex, and an initial transcribing complex in which short RNA chains are synthesized and released prior to promoter clearance. Activation of transcription requires acceleration of the rate-limiting step of this pathway. Many activator proteins bind to one or more sites upstream of the promoter and make specific contacts with the α subunit of RNAP (2). The resulting protein–protein interactions may stimulate holoenzyme binding (3), DNA-melting (4), or promoter clearance (5), thereby leading to activation. Other transcriptional activators contact the σ subunit of RNAP (6–8).
Sigma factors belonging to the large σ70 family contain several highly conserved regions. Biochemical and genetic data implicate regions 4.2 and 2 in recognition of the −35 and −10 promoter elements, respectively (for review see ref. 1). The three-dimensional structure of a proteolytic fragment of σ70 reveals that regions 2.3 and 2.4 contribute to an amphipathic α-helix containing exposed residues implicated in −10 recognition and DNA melting (9–11).
RNAP contacts between 60 and 90 bp of DNA when bound at a promoter (12). Although much of the specificity for binding resides in the recognition by σ of the highly conserved −10 and −35 elements, other sequences can significantly influence promoter strength (13–18). For example, the Bacillus subtilis spoVG promoter has an extended architecture and requires an AT-rich region between −40 and −70 for high activity in vivo and in vitro (19, 20). A similar upstream promoter element has been defined at Escherichia coli rrnB P1. The rrnB P1 upstream promoter (UP) element extends from about −40 to −60, stimulates transcription in vivo and in vitro, and binds to the carboxyl-terminal domain (CTD) of the α subunit of RNA polymerase (21–25). Analogous experiments have demonstrated that high-level transcription of the flagellin gene (hag) in B. subtilis also requires an UP element that interacts with α (26).
One can envision two formal models to explain how the hag UP element activates transcription. In model 1, the α:UP interaction contributes in an additive fashion to the first step of transcription initiation, holoenzyme binding to promoter DNA. In model 2, the α:UP interaction affects σ to stimulate either promoter recognition or DNA melting. Because E. coli σ70 and αCTD can be crosslinked in solution (27) and bind to adjacent promoter elements (22), an interaction between α and σ during UP element activation is plausible. Interestingly, although the principal effect of the rrnB P1 UP element on initiation is to enhance binding (21), when placed next to λ PRM this same element activates a subsequent step in the initiation pathway (28).
In this study, we use heteroduplex (bubble) templates to demonstrate that the UP element can activate the flagellin promoter (Phag) in the presence or absence of the cognate σD factor. In the course of this analysis we observed complex effects of σ on start-site selection. Comparison of the effects of three different σ factors on start-site selection from these bubble templates supports a model in which the σ subunit recognizes the −10 promoter region by sequence-specific interactions with the nontemplate strand and thereby acts to position the catalytic site on the template strand.
MATERIALS AND METHODS
Preparation of DNA Templates for in Vitro Transcription.
Bubble templates (Fig. 1) contained either the wild-type nontemplate strand (wtNT) or the wild-type template strand (wtT). Each position in the heteroduplex region contained the identical rather than the complementary base on each strand. Templates contained hag promoter DNA extending upstream to either −65 (+UP) or −43 (−UP) and were made using PCR and oligonucleotide primers containing or lacking 5′ biotin modification as described (29). Briefly, wild-type and mutant PCR products, each biotin-labeled on one strand, were denatured and reannealed and separated by agarose gel electrophoresis after prebinding Streptavidin (GIBCO/BRL). Unbound heteroduplex DNA was gel purified using Qiaquick system (Qiagen). Biotinylated oligos were >92% homogeneous as assayed by gel mobility shift when bound to Streptavidin. Homoduplex transcription templates were made by PCR with nonbiotinylated primers and gel purified using the Qiaquick system.
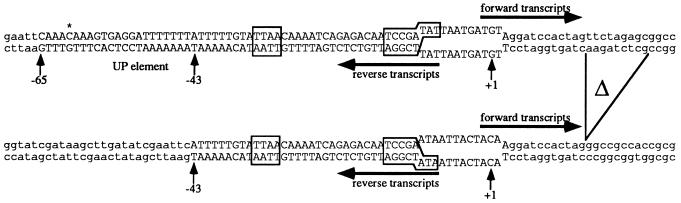
Figure 1.
Promoter regions of two of the four bubble templates used in this study. The +UP template shown contains the wild-type nontemplate strand (wtNT) in the heteroduplex region (Upper), and the −UP template contains the wild-type template strand (wtT) (Lower). Boxes highlight the −10 and −35 regions of the hag promoter. Uppercase letters represent hag DNA and lowercase letters represent vector DNA. The A-61C mutation is indicated by an asterisk. The (−UP) templates have a 9-bp deletion (indicated by Δ) to resolve RNA products in mixed transcription experiments. Note that the vector DNA extends beyond the regions shown such that the the full-length templates are 340 bp (−UP) and 370 bp (+UP).
PCR products were generated from promoter-containing plasmids using primers 5′-CGATTATGTTGGGTAACGCCAGG-3′ and 5′-TCCGGCTCCTATGTTGTGTGGAA-3′, which anneal to pBluescript KSII(+) (Stratagene) on either side of the polylinker. Promoter fragments, containing wild-type or substituted sequence from −10 to +2, were generated by extending annealed oligos as described (30). Forward oligos F(+UP) (5′-CGGAATTCAAACAAAGTGAGGATTTTTTTATTTTTGTATTAACAAAATCAGAGACAATC-3′) or F (−UP) (5′-CGGAATTCATTTTTGTATTAACAAAATCAGAGACAATC-3′) and reverse oligos B12 (5′-CGCGGATCCTTGTAGTAATTATTCGGATTGTCTCTGATTT-3′) or R1 (5′-CGCGGATCCTACATCATTAATATCGGATTGTCTCTGATTT-3′) were annealed in each possible combination, extended, cloned into pBluescript KSII(+) via the EcoRI and BamHI sites, and verified by DNA sequencing. The F(+UP) oligonucleotide contains an A-61C base substitution to allow efficient synthesis. Plasmids containing −UP promoters were digested with SpeI and EagI, rendered blunt-ended by treatment with Sequenase and dNTPs, and religated to generate a 9-bp downstream deletion to allow resolution of +UP and −UP transcripts.
In Vitro Transcription Reactions.
B. subtilis core enzyme (lacking δ) was purified as described (26). The δ subunit does not alter Phag UP activation (unpublished data). Purification of σA and σD were described (10, 31), and purification of σX has not yet been presented (X. Huang and J.D.H., unpublished data). Holoenzymes were reconstituted by incubating core with a 5-fold molar excess of σ on ice for >3 min.
Run-off transcription reactions were performed in 30 μl of transcription buffer (18 mM Tris⋅HCl, pH 8.0/10 mM MgCl2/10–13% (vol/vol) glycerol/8 mM 2-mercaptoethanol/100 μg BSA per ml/100 μM [α-32P]UTP/800 μM each CTP, ATP, and GTP) with 0.2 pmol template (0.1 pmol of each template in competition assays) and NaCl added as indicated. Reactions were initiated by adding prewarmed RNAP to a final concentration of 90 nM. After incubation at 25°C for the indicated time, reactions were stopped by addition to 100 μl stop solution (2.5 M NH4OAc/20 mM EDTA/5 μg carrier DNA or 20 μg glycogen), extracted with phenol/CHCl3, and ethanol precipitated. Products were resolved by 6% denaturing PAGE and quantified using a Molecular Dynamics PhosphoImaging system and imagequant software.
Primer Extension Mapping.
In vitro RNA was generated as above at 150 mM NaCl except that UTP was unlabeled and at 800 μM. After 12 min, the RNA was phenol/CHCl3 extracted, and 2 pmol of 5′-[32P]AATACGACTCACTATAG-3′ or 5′-[32P]AAACAGCTATGACCATG-3′ (50,000 cpm per pmol) was added for detection of forward or reverse transcripts, respectively. Glycogen (20 μg) was added with NH4OAc to 2.5 M, and the nucleic acids were ethanol precipitated. The pellet was washed with 70% ethanol and dissolved in 30 μl of hybridization solution (60 mM NaCl/50 mM Tris⋅HCl, pH 8.0/10 mM DTT), heated to 90°C for 3 min, and cooled slowly. The annealed mixture (20 μl) was added to 10 μl of extension solution containing avian myeloblastosis virus reverse transcriptase and dNTPs according to the manufacturer’s instructions (Promega). After 30 min at 37°C, the extension reactions were phenol extracted, and the products were resolved by 6% denaturing PAGE. A plasmid containing an appropriate promoter variant was sequenced using the identical primer and electrophoresed in adjacent lanes.
RESULTS AND DISCUSSION
We have engineered heteroduplex (bubble) templates (Fig. 1) to address the role of σD in transcription activation by the flagellin UP element. We hypothesized that if the primary effect of the UP element was to enhance DNA melting, then UP stimulation would be ineffective on DNA templates in which the start-site region was single stranded by virtue of a 12-bp DNA mismatch. Similar heteroduplex templates have been employed previously to test the effect of σA region 2.3 mutations on in vitro transcription (32), to determine the role of ATP hydrolysis in initiation by RNA polymerase II (33), and to study the mechanism of activation by E. coli NtrC (34).
The Flagellin UP Element Stimulates Transcription from Bubble Templates in the Presence or Absence of σD.
To facilitate the design of appropriate bubble templates, it was first necessary to confirm that the minimal UP element defined in vivo could function in vitro. We have shown previously that high-level transcription from Phag in vivo requires sequences upstream to position −65 (26). This promoter construct (Phag-65) contains a single base substitution (A-61C) that disrupts a 7-bp A-tract but does not affect in vivo expression, and this mutation was included in the present study for technical reasons (see Materials and Methods). This minimal UP element (−43 to −65) activates transcription in vitro (Fig. 2A): RNA products generated from the +UP template predominate when Phag-65 (+UP) is competed with Phag-43 (−UP) at elevated salt concentration. The salt dependence of the UP element stimulation was expected based on previous work (26).
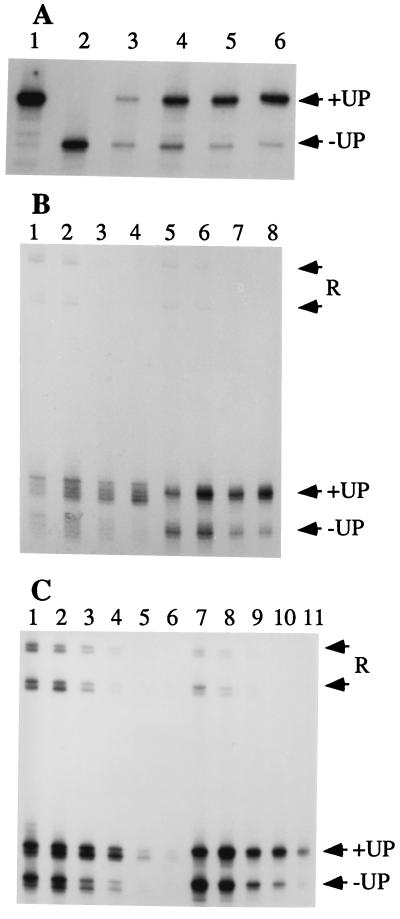
Figure 2.
In vitro run-off transcription. (A) Transcription from homoduplex templates. RNA products generated from Phag(+UP) (lane 1), Phag(-UP) (lane 2), or both templates competing (lanes 3–6). Reactions contained NaCl at 100 mM (lanes 1, 2, and 4), 50 mM (lane 3), 150 mM (lane 5), or 200 mM (lane 6). (B) Transcription from competing +UP and −UP wtNT bubble templates. RNA products were generated by core (lanes 1–4) or σD-holoenzyme (lanes 5–8) in transcription reactions with NaCl at 100 mM (lanes 1 and 5), 150 mM (lanes 2 and 6), 200 mM (lanes 3 and 7), or 250 mM (lanes 4 and 8). Forward and reverse (R) transcripts derived from the +UP templates migrate more slowly than those from the −UP templates. (C) Transcription from competing +UP and −UP wtT bubble templates. RNA products were generated by core (lanes 1–6) or σD-holoenzyme (lanes 7–11) in transcription reactions with NaCl at 100 mM (lanes 1 and 7), 150 mM (lanes 2 and 8), 200 mM (lanes 3 and 9), 250 mM (lanes 4 and 10), 300 mM (lanes 5 and 11), or 350 mM (lane 6).
We used bubble templates that contain a 12-bp region of noncomplementarity (−10 to +2; Fig. 1), designed to mimic the strand-separated DNA in the mature open complex at Phag (35). These templates contain either the wtNT or wtT sequence within the bubble region. Consistent with previous studies of E. coli core enzyme (36), B. subtilis core initiates from these bubble templates in both directions, yielding RNA products of ≈140 nt (forward transcripts) and ≈230 nt (reverse transcripts) (Fig. 2 B and C). B. subtilis core is inactive on homoduplex hag promoter DNA, demonstrating a lack of detectable σD activity (Fig. 3).
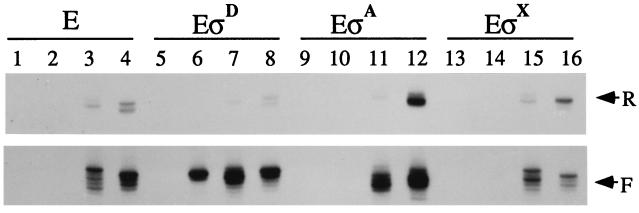
Figure 3.
In vitro transcripts produced from homoduplex and bubble templates by core (E), EσD, EσA, or EσX (as indicated) in reactions containing 150 mM NaCl. All templates contain the UP element. Promoter variants in each set of four include the homoduplex mutant containing substituted sequence from −10 to +2 (lanes 1, 5, 9, and 13), the homoduplex wild type (lanes 2, 6, 10, and 14), the wtNT bubble (lanes 3, 7, 11, and 15), and the wtT bubble (lanes 4, 8, 12, and 16).
We anticipated that the UP element might orient core on these templates to favor forward transcription. In fact, core does produce primarily forward transcripts, but this effect is noted for both +UP and −UP templates (Fig. 2 B and C). Therefore, a feature of these templates other than the UP element seems to confer a preferred direction of initiation. Although the α:UP element interaction does not appear to play a major role in orienting transcription from these bubble templates, addition of σD does suppress the production of reverse transcripts (Fig. 2C).
To determine whether the UP element could stimulate transcription by core, we have used competition run-off transcription assays (Fig. 2 B and C). Significantly, the UP element stimulates both σD-holoenzyme (EσD) and core (E) on these bubble templates. Optimal UP-dependent stimulation requires high salt conditions (as with homoduplexes) and is about 80% of that observed on homoduplexes (Fig. 2). As expected, the reverse transcripts are not affected by the presence or absence of the UP element. These data demonstrate that the UP element can stimulate transcription independent of both σ and the DNA-melting process. Electrophoretic mobility shift assays demonstrate that the UP element increases the affinity of RNAP for DNA even in the absence of NTP substrates (26), consistent with stimulation of an early step in the initiation pathway. Together with the observations that UP element activation is maximal at high salt and low concentrations of RNAP, these data argue that the UP element is stimulating the initial binding of RNAP to these bubble templates. Analysis of the rrnB P1 UP element also suggested that the primary stimulatory effect was on binding (21), but when appended to the λ PRM core promoter, this element acts on a later step in initiation (28).
σD Restricts Forward Transcription Start Sites on These Bubble Templates.
The ability of both core and holoenzyme to initiate transcription from bubble templates provides a unique experimental system to observe effects of σ on transcript initiation. Interactions between σ and the conserved −35 and −10 elements are critical for promoter recognition, and σ also helps catalyze the DNA strand separation that leads to the formation of open complexes (11). In the course of the above experiments, we observed an unexpected σ-dependent variability in start-site selection. To analyze this effect in more detail, we used run-off transcription and primer extension experiments to assign the transcription start points used by core, EσD, EσA, and EσX (Figs. 3 and 4).
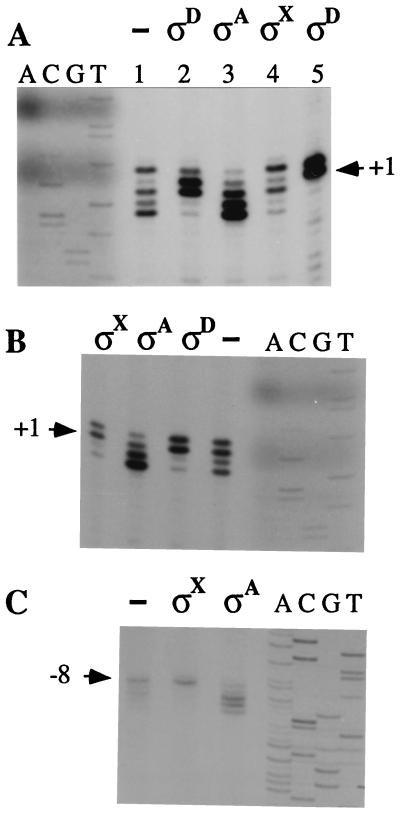
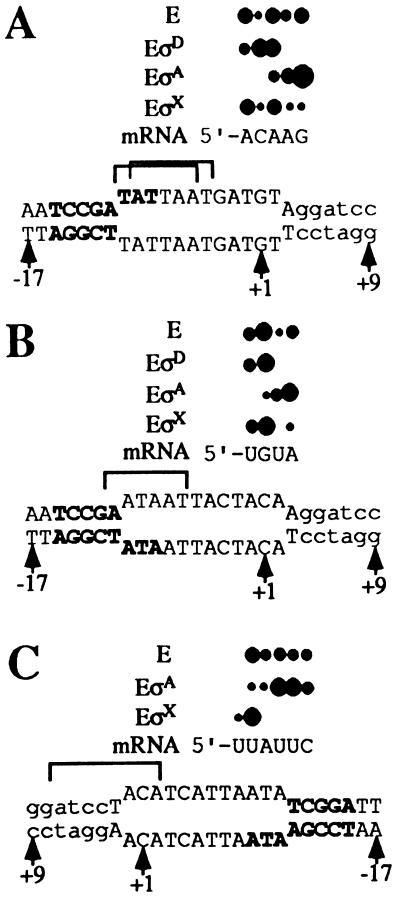
Figure 4.
Primer extension analysis of bubble transcripts. The primer extension products represent forward transcripts from the wtNT bubble template (lanes 1–4) or the Phag homoduplex (lane 5) generated by core in the presence of no added σ (−), σD, σA, or σX as indicated (A); forward transcripts from the wtT bubble template generated by core alone (−) or in the presence of σX, σA, or σD (B); and reverse transcripts from the wtT bubble template by core alone (−), or in the presence of σX or σA as indicated (C). Reference positions are indicated based on comigration of sequencing lanes (A, C, G, T).
B. subtilis core generally shows preferential initiation with purine nucleotides, similar to that described for holoenzyme in vivo (37–39). Core initiates forward transcripts from the wtNT bubble template mostly with purines at −1, +2, +3, and +4, whereas initiation with CTP at +1 is relatively infrequent (Fig. 4A). In contrast, EσD initiates only near the natural start sites, and initiation is now frequent at +1, even though this requires the use of CTP as initiating substrate. Similar results are observed with the wtT bubble template (Fig. 4B): core initiates predominantly at positions −1, +1, and +3, and less frequently with U at +2. In contrast, EσD initiates almost exclusively with U and G at −1 and +1, a pattern nearly identical to EσD start sites from homoduplex Phag template (Fig. 4A, lane 5). Thus, σD restricts start sites to near +1 on both bubble templates even when this requires use of a nonpreferred initiating substrate.
The ability of σD to affect start-site selection is presumably due to the recognition of either the −35 region, the −10 region, or both. It is interesting to note, however, that EσD is inactive on the mutant homoduplex, in which the −10 sequence is altered from 5′-TCCGATAT-3′ to 5′-TCCGAATA-3′ (Fig. 3, lane 5). This is consistent with the observation that all σD-dependent promoters contain the invariant −10 region sequence, 5′-CGATA-3′ (40–49). Because promoter melting at Phag nucleates with a localized distortion of the central AT base step (35), the inability of EσD to initiate on the mutant homoduplex may be due to a melting defect.
Start-Site Selection by EσA Implicates a Role for Single-Stranded DNA in −10 Recognition.
To assess whether the σD-dependent effects on start-site selection were due to the presence of the cognate Phag recognition sequences, or to a general conformational change elicited by σ-core binding, we studied the effect of σA on initiation from these templates. Note that neither homoduplex is transcribed by EσA (Fig. 3, lanes 9 and 10) despite the presence of a possible −35 sequence 5′-TTaACA-3′ (matches to consensus in uppercase) and possible −10-like sequences (see below). As expected, EσA is able to produce forward transcripts from both bubble templates. Surprisingly, EσA stimulates and restricts initiation at and near position +4 on the wtNT bubble template (Fig. 4A). Because the nontemplate strand sequence 5′-TATTAAT-3′ is positioned from −9 to −4 in the heteroduplex region, an appropriate distance from +4, we suggest that bases within this sequence may be specifically recognized by σA (Fig. 5A). This sequence resembles the σA −10 consensus (TATAAT), except that it contains an additional internal T: within this TATTAAT sequence the hexanucleotides 5′-TATtAa-3′ and 5′-atTAAT-3′ both match consensus at 4 of 6 positions. EσA also enhances transcript initiation near +2 and +3 on the wtT bubble template (Figs. 4B and 5B). In this case, the nontemplate strand contains the sequence 5′-aATAAT-3′ with a 5 of 6 match to consensus, which may explain the downstream shift (relative to core alone) in preferred initiation sites. There is no apparent correlation between start-site selection and −10-like sequences on the template strand: although both bubble templates contain sequences similar to the template strand consensus (3′-ATATTA-5′), their locations predict that initiation on the wtT template (in response to 3′-taATTA-5′) should be shifted downstream by 2 nt relative to the wtNT template (in response to 3′-tTATTA-3′), the opposite of the observed effect.
Figure 5.
Summary of the primer extension data from Fig. 4. Bases of the Phag −10 element are shown in bold, and start-site positions are indicated by solid circles, where relative size indicates relative initiation frequency. Bracketed sequences display −10-like sequences of the nontemplate strand that may be recognized by EσA (A and B) or EσX (C). Note that the wtT bubble in C is inverted for comparison purposes.
These data suggest that σA can respond to sequences on the nontemplate strand and thereby influence start-site selection, even when these same sequences do not support initiation on homoduplex templates. Several other recent results indicate that σ can recognize the −10 nontemplate sequence as single-stranded DNA. Initiation from promoters containing single-base mismatches reveals that Eσ70 selectively recognizes the nontemplate base at −11 (50). In addition, holoenzyme, but not core, binds selectively to oligonucleotides containing the −10 consensus nontemplate sequence (ref. 51; X. Huang and J.D.H., unpublished results).
Effects of σX and σA on Reverse Transcript Initiation.
Holoenzyme containing the alternative σ factor σX influences forward transcript initiation by modestly suppressing initiation from downstream positions (Fig. 4 A and B). Also, EσX displays an enhanced reverse transcript from the wtT bubble template (Fig. 3, lane 16). A similar enhancement of reverse transcription is observed on this template by EσA (lane 12) but not by EσD. Primer extension mapping of these reverse transcripts reveals that core initiates from positions −8, −9, −10, −11, and −12 (Fig. 4C). Addition of σX restricts transcription almost exclusively to −8. Because only one σX-dependent promoter has been characterized (X. Huang and J.D.H., unpublished results), consensus −10 and −35 sequences are not yet known. But intriguingly, the known PX contains a sequence (5′-ATACGACA-3′) 8 base pairs upstream of its start site, and a similar sequence (5′-ATcCtACA-3′) occurs 7 base pairs upstream of this σX-enhanced bubble start site (Fig. 5C). We suggest that σX may be affecting reverse transcription by recognizing this −10-like sequence. In the presence of σA, reverse transcript initiation from the wtT bubble is enhanced at −10 and −11 but suppressed at −8 (Fig. 4C). A 5′-AT-3′ on the nontemplate strand is positioned 7 or 8 nucleotides upstream of these starts (−1 to −2) and may be involved in the observed stimulation.
Conclusions.
We have used bubble templates incorporating features of the σD-dependent flagellin promoter to compare transcript initiation by core and holoenzyme directly. Our analyses demonstrate that UP element stimulation occurs efficiently in the absence of σD and supports a model in which the UP element acts by enhancing the initial association of RNAP with DNA. Our results exclude models in which UP element stimulation is obligatorily mediated through σ. Thus, enhanced promoter binding in the presence of the UP element is not due to an increased interaction of holoenzyme with the −10 or −35 elements, or to an increased rate of DNA melting.
Although σ is not required for UP element-mediated activation in vitro, σ is clearly important for several other steps in transcription initiation. The role of σ in recognition of the −35 and −10 elements is well established. Recently, it has been demonstrated by direct binding studies that σ acts to sense the relative spacing of these key promoter elements (52). σ also participates directly in the DNA-melting step, and we have identified aromatic amino acids thought to interact with the nontranscribed strand (10, 53). In several cases, mutations that impair DNA melting also affect promoter selectivity. Hence, we suggested that the −10 region might be recognized, at least in part, as single-stranded DNA (10). The effects of σ on start-site selection from bubble templates support this model.
In addition to the above biochemical activities, our analysis of bubble templates indicate that σ is a major determinant of start-site localization. σD restricts transcription initiation to near the natural start site, presumably in response to specific interaction with the −10 element. Similarly, σA restricts initiation to sites near +4, and these start sites correlate with the presence of −10-like elements on the nontranscribed strand. Thus, we suggest that the contacts of σ with the −10 region are rigidly positioned relative to the catalytic center of RNAP in preinitiation complexes. In contrast, the catalytic center of RNAP is often flexible relative to other parts of the enzyme during initiation and elongation (54–58). Start-site selection reflects a competition between those sites at a preferred distance from the −10 element and those sites that allow initiation with a purine. This competition can be influenced, both in vitro and in vivo, by the relative availability of NTPs. Indeed, altering start-site selection by as little as a single nucleotide contributes to the regulation of several pyrimidine biosynthesis operons in E. coli (39, 59, 60). In addition to its possible regulatory implications, the observation that σ can restrict start sites on bubble templates provides a new assay for single-stranded DNA recognition by σ, independent of the need for start-site melting.
Acknowledgments
We thank Chris Roberts for advice on the preparation of bubble templates, Xuejun Huang for purified σA and σX, and J. Calvo and V. J. Stewart for helpful comments on the manuscript. This research was supported by National Institutes of Health Grant GM47446.
ABBREVIATIONS
- RNAP
RNA polymerase
- UP
upstream promoter
- wtNT
wild-type nontemplate strand
- wtT
wild-type template strand
References
- 1.Helmann J D. In: Transcription: Mechanisms and Regulation. Conaway R C, Conaway J W, editors. Vol. 3. New York: Raven; 1994. pp. 1–17. [Google Scholar]
- 2.Ishihama A. J Bacteriol. 1993;175:2483–2489. doi: 10.1128/jb.175.9.2483-2489.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malan T P, Kolb A, Buc H, McClure W R. J Mol Biol. 1984;180:881–909. doi: 10.1016/0022-2836(84)90262-6. [DOI] [PubMed] [Google Scholar]
- 4.Herbert M, Kolb A, Buc H. Proc Natl Acad Sci USA. 1986;83:2807–2811. doi: 10.1073/pnas.83.9.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menendez M, Kolb A, Buc H. EMBO J. 1987;6:4227–4234. doi: 10.1002/j.1460-2075.1987.tb02771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li M, Moyle H, Susskind M M. Science. 1994;263:75–77. doi: 10.1126/science.8272867. [DOI] [PubMed] [Google Scholar]
- 7.Kumar A, Grimes B, Fujita N, Makino K, Malloch R A, Hayward R S, Ishihama A. J Mol Biol. 1994;235:405–413. doi: 10.1006/jmbi.1994.1001. [DOI] [PubMed] [Google Scholar]
- 8.Baldus J M, Buckner C M, Moran C P., Jr Mol Microbiol. 1995;17:281–290. doi: 10.1111/j.1365-2958.1995.mmi_17020281.x. [DOI] [PubMed] [Google Scholar]
- 9.Malhotra A, Severinova E, Darst S A. Cell. 1996;87:127–136. doi: 10.1016/s0092-8674(00)81329-x. [DOI] [PubMed] [Google Scholar]
- 10.Juang Y, Helmann J D. J Mol Biol. 1994;235:1470–1488. doi: 10.1006/jmbi.1994.1102. [DOI] [PubMed] [Google Scholar]
- 11.deHaseth P L, Helmann J D. Mol Microbiol. 1995;16:817–824. doi: 10.1111/j.1365-2958.1995.tb02309.x. [DOI] [PubMed] [Google Scholar]
- 12.Ozoline O N, Tsyganov M A. Nucleic Acids Res. 1995;23:4533–4541. doi: 10.1093/nar/23.22.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McAllister C F, Achberger E C. J Biol Chem. 1989;264:10451–10456. [PubMed] [Google Scholar]
- 14.Bracco L, Kotlarz D, Kolb A, Diekmann S, Buc H. EMBO J. 1989;8:4289–4296. doi: 10.1002/j.1460-2075.1989.tb08615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu L M, Giannini J K, Leung T C, Crosthwaite F C. Biochemistry. 1991;30:813–822. doi: 10.1021/bi00217a035. [DOI] [PubMed] [Google Scholar]
- 16.Sander P, Langert W, Mueller K. J Biol Chem. 1993;268:16907–16916. [PubMed] [Google Scholar]
- 17.Hirota Y, Ohyama T. J Mol Biol. 1995;254:566–578. doi: 10.1006/jmbi.1995.0639. [DOI] [PubMed] [Google Scholar]
- 18.Giladi H, Murakami K, Ishihama A, Oppenhein A B. J Mol Biol. 1996;260:484–491. doi: 10.1006/jmbi.1996.0416. [DOI] [PubMed] [Google Scholar]
- 19.Banner C D B, Moran C P, Jr, Losick R. J Mol Biol. 1983;168:351–365. doi: 10.1016/s0022-2836(83)80023-0. [DOI] [PubMed] [Google Scholar]
- 20.Frisby D, Zuber P. J Bacteriol. 1991;173:7557–7564. doi: 10.1128/jb.173.23.7557-7564.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rao L, Ross W, Appleman J A, Gaal T, Leirmo S, Schlax P J, Record M T, Jr, Gourse R L. J Mol Biol. 1994;235:1421–1435. doi: 10.1006/jmbi.1994.1098. [DOI] [PubMed] [Google Scholar]
- 22.Ross W, Gosink K K, Salomon J, Igarashi K, Zou C, Ishihama A, Severinov K, Gourse R L. Science. 1993;262:1407–1413. doi: 10.1126/science.8248780. [DOI] [PubMed] [Google Scholar]
- 23.Blatter E E, Ross W, Tang H, Gourse R L, Ebright R H. Cell. 1994;78:889–896. doi: 10.1016/s0092-8674(94)90682-3. [DOI] [PubMed] [Google Scholar]
- 24.Jeon Y H, Negishi T, Shirakawa M, Yamazaki T, Fujita N, Ishihama A, Kyogoku Y. Science. 1995;270:1495–1497. doi: 10.1126/science.270.5241.1495. [DOI] [PubMed] [Google Scholar]
- 25.Gaal T, Ross W, Blatter E E, Tang H, Jia X, Krishnan V V, Assa-Munt N, Ebright R H, Gourse R L. Genes Dev. 1996;10:16–26. doi: 10.1101/gad.10.1.16. [DOI] [PubMed] [Google Scholar]
- 26.Fredrick K, Caramori T, Chen Y F, Galizzi A, Helmann J D. Proc Natl Acad Sci USA. 1995;92:2582–2586. doi: 10.1073/pnas.92.7.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMahan S, Burgess R R. Biochemistry. 1994;33:12092–12099. doi: 10.1021/bi00206a012. [DOI] [PubMed] [Google Scholar]
- 28.Tang Y, Murakami K, Ishihama A, deHaseth P L. J Bacteriol. 1996;178:6945–6951. doi: 10.1128/jb.178.23.6945-6951.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ring B Z, Yarnell W S, Roberts J W. Cell. 1996;86:485–493. doi: 10.1016/s0092-8674(00)80121-x. [DOI] [PubMed] [Google Scholar]
- 30.Reidharr-Olson J, Bowie J U, Breyer R M, Hu J C, Knight K L, Lim W A, Mossing M C, Parsell D A, Shoemaker K R, Sauer R T. In: Methods in Enzymology. Sauer R T, editor. Vol. 208. San Diego: Academic; 1991. pp. 564–586. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, Helmann J D. J Mol Biol. 1995;249:743–753. doi: 10.1006/jmbi.1995.0333. [DOI] [PubMed] [Google Scholar]
- 32.Aiyar S E, Juang Y, Helmann J D, deHaseth P L. Biochemistry. 1994;33:11501–11506. doi: 10.1021/bi00204a012. [DOI] [PubMed] [Google Scholar]
- 33.Tantin D, Carey M. J Biol Chem. 1994;269:17397–17400. [PubMed] [Google Scholar]
- 34.Wedel A, Kustu S. Genes Dev. 1995;9:2042–2052. doi: 10.1101/gad.9.16.2042. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y-F, Helmann J D. J Mol Biol. 1997;267:47–59. doi: 10.1006/jmbi.1996.0853. [DOI] [PubMed] [Google Scholar]
- 36.Aiyar S E, Helmann J D, deHaseth P L. J Biol Chem. 1994;269:13179–13184. [PubMed] [Google Scholar]
- 37.Hawley D K, McClure W R. Nucleic Acids Res. 1983;11:2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Helmann J D. Nucleic Acids Res. 1995;23:2351–2360. doi: 10.1093/nar/23.13.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu J, Turnbough C L., Jr J Bacteriol. 1994;176:2938–2945. doi: 10.1128/jb.176.10.2938-2945.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mirel D B, Chamberlin M J. J Bacteriol. 1989;171:3095–3101. doi: 10.1128/jb.171.6.3095-3101.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mirel D B, Lustre V M, Chamberlin M J. J Bacteriol. 1992;174:4197–4204. doi: 10.1128/jb.174.13.4197-4204.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lazarevic V, Margot P, Soldo B, Karamata D. J Gen Microbiol. 1992;138:1949–1961. doi: 10.1099/00221287-138-9-1949. [DOI] [PubMed] [Google Scholar]
- 43.Chen L, Helmann J D. J Bacteriol. 1994;176:3093–3101. doi: 10.1128/jb.176.11.3093-3101.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fredrick K, Helmann J D. J Bacteriol. 1994;176:2727–2735. doi: 10.1128/jb.176.9.2727-2735.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mirel D B, Lauer P, Chamberlin M J. J Bacteriol. 1994;176:4492–4500. doi: 10.1128/jb.176.15.4492-4500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perego M, Glaser P, Minutello A, Strauch M A, Leopold K, Fischer W. J Biol Chem. 1995;270:15598–15606. doi: 10.1074/jbc.270.26.15598. [DOI] [PubMed] [Google Scholar]
- 47.Ogura M, Tanaka T. J Bacteriol. 1996;178:216–222. doi: 10.1128/jb.178.1.216-222.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanlon D W, Márques-Magaña L, Carpenter P B, Chamberlin M J, Ordal G W. J Biol Chem. 1992;267:12055–12060. [PubMed] [Google Scholar]
- 49.Hanlon D W, Rosario M L, Ordal G W, Venema G, Sinderen D V. Microbiology. 1994;140:1847–1854. doi: 10.1099/13500872-140-8-1847. [DOI] [PubMed] [Google Scholar]
- 50.Roberts C W, Roberts J W. Cell. 1996;86:495–501. doi: 10.1016/s0092-8674(00)80122-1. [DOI] [PubMed] [Google Scholar]
- 51.Severinova E, Severinov K, Fenyö D, Marr M, Brody E N, Roberts J W, Chait B T, Darst S A. J Mol Biol. 1996;263:637–647. doi: 10.1006/jmbi.1996.0604. [DOI] [PubMed] [Google Scholar]
- 52.Dombroski A J, Johnson B D, Lonetto M, Gross C. Proc Natl Acad Sci USA. 1996;93:8858–8862. doi: 10.1073/pnas.93.17.8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Juang Y, Helmann J D. Biochemistry. 1995;34:8465–8473. doi: 10.1021/bi00026a030. [DOI] [PubMed] [Google Scholar]
- 54.Mustaev A, Kashlev M, Zaychikovi E, Grachevi M, Goldfarb A. J Biol Chem. 1993;268:19185–19187. [PubMed] [Google Scholar]
- 55.Nudler E, Goldfarb A, Kashlev M. Science. 1994;265:793–796. doi: 10.1126/science.8047884. [DOI] [PubMed] [Google Scholar]
- 56.Nudler E, Kashlev M, Nikiforov V, Goldfarb A. Cell. 1995;81:351–357. doi: 10.1016/0092-8674(95)90388-7. [DOI] [PubMed] [Google Scholar]
- 57.Wang D, Meier T I, Chan C L, Feng G, Lee D N, Landick R. Cell. 1995;81:341–350. doi: 10.1016/0092-8674(95)90387-9. [DOI] [PubMed] [Google Scholar]
- 58.Chamberlin M J. The Harvey Lectures. Vol. 88. New York: Wiley; 1995. pp. 1–21. [PubMed] [Google Scholar]
- 59.Wilson H R, Archer C D, Liu J, Turnbough C L., Jr J Bacteriol. 1992;174:514–524. doi: 10.1128/jb.174.2.514-524.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qi F, Turnbough C L., Jr J Mol Biol. 1995;254:552–565. doi: 10.1006/jmbi.1995.0638. [DOI] [PubMed] [Google Scholar]