Abstract
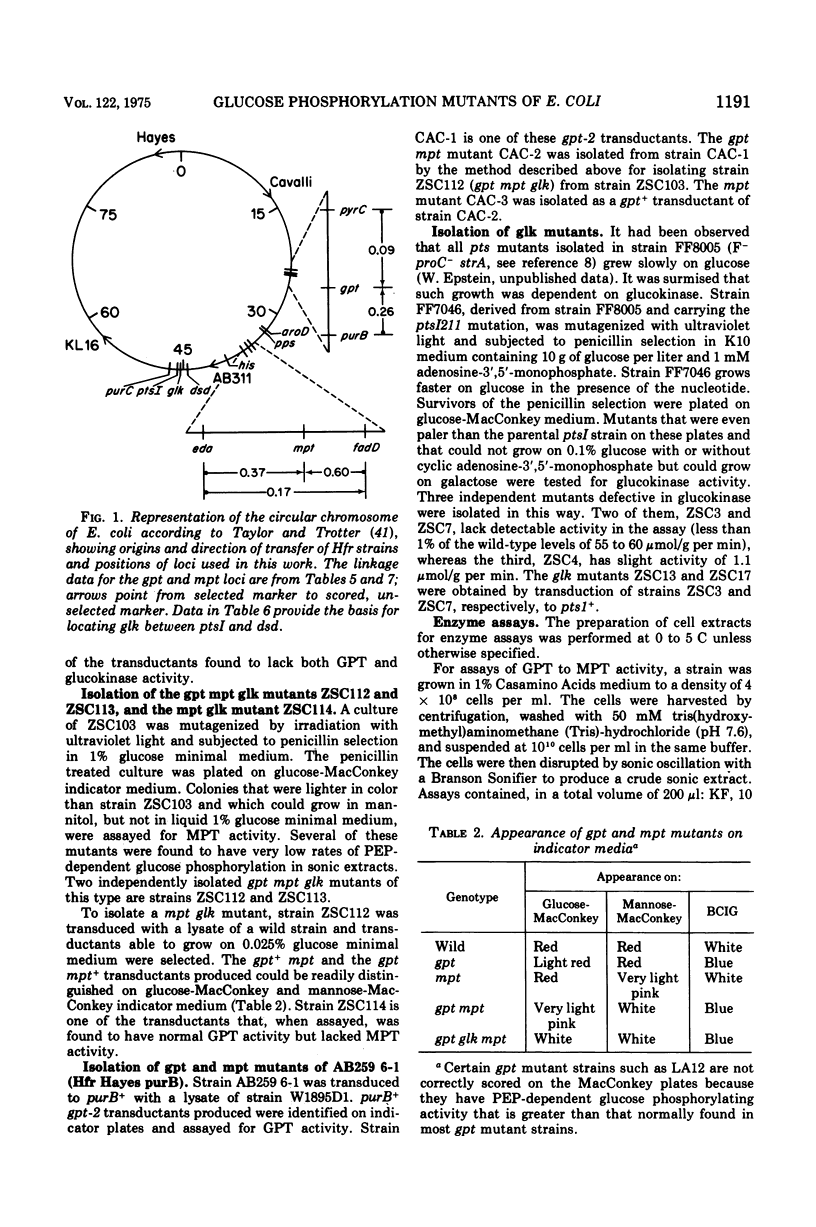
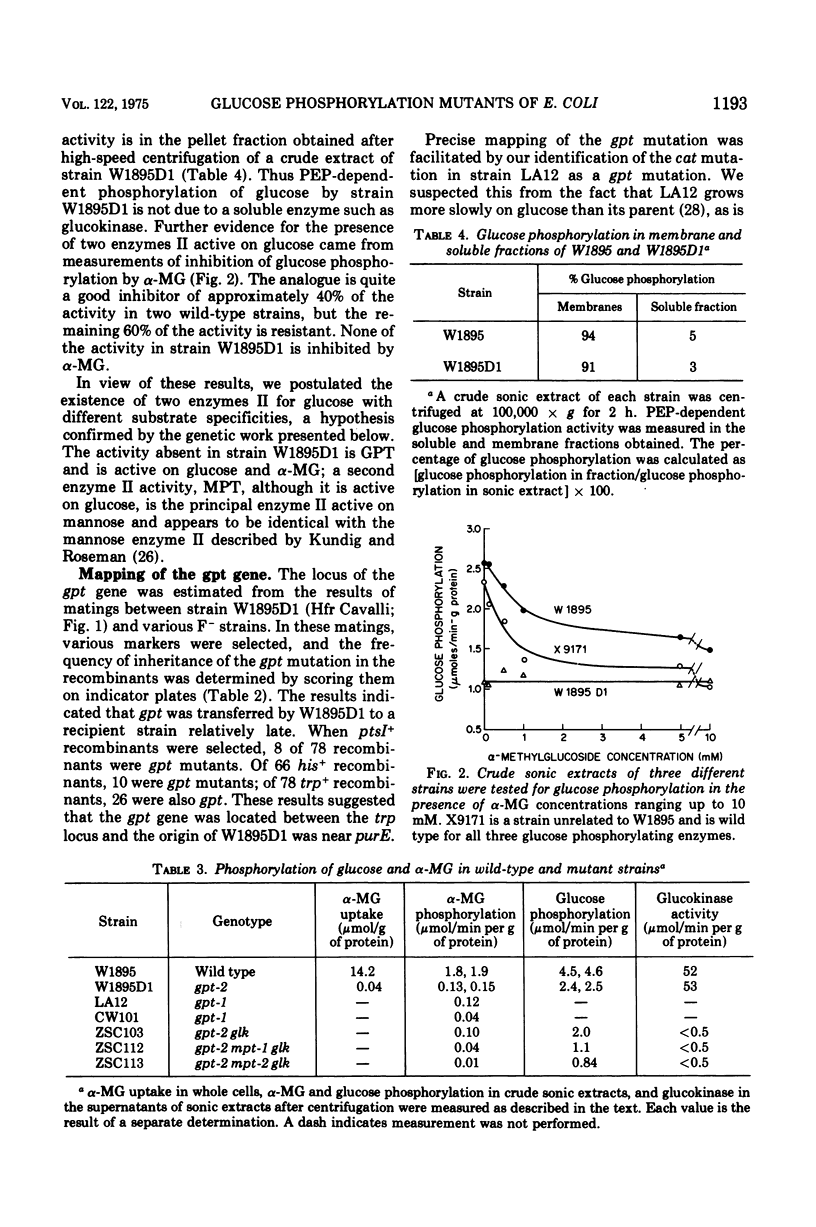
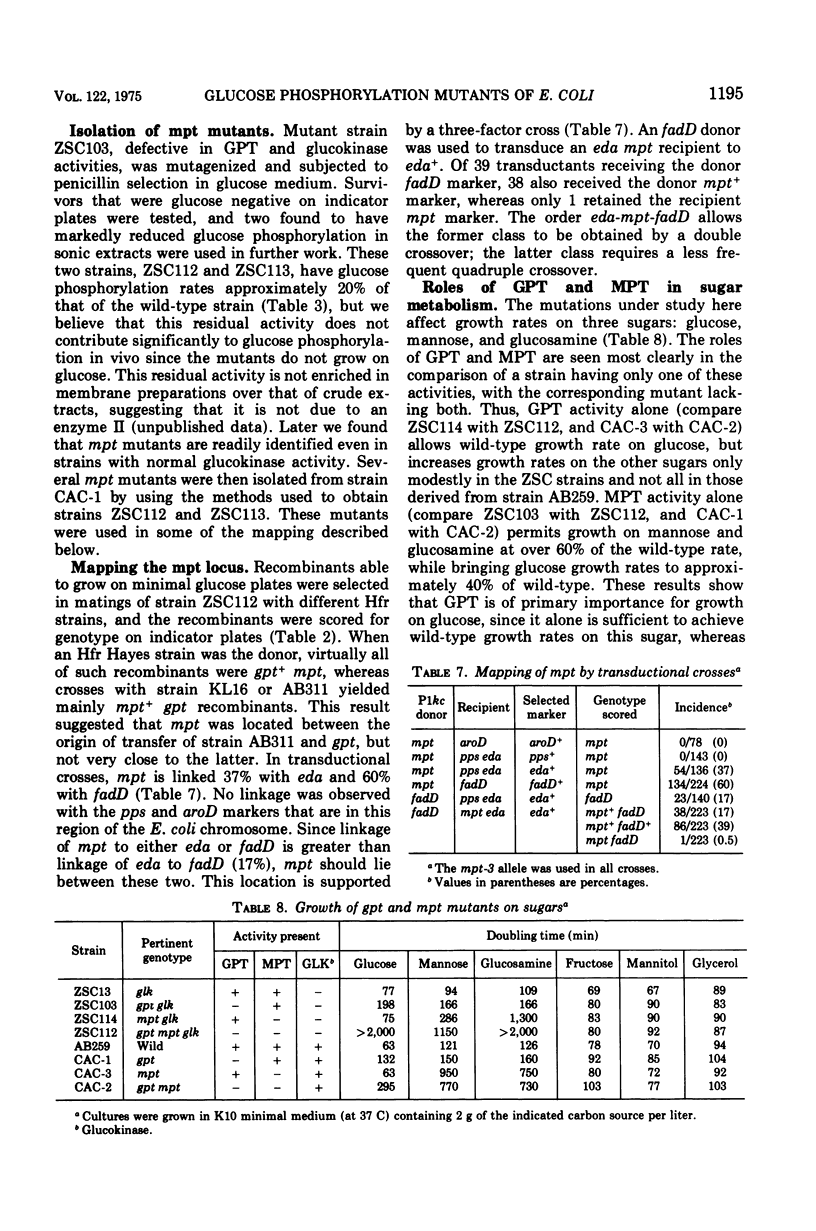
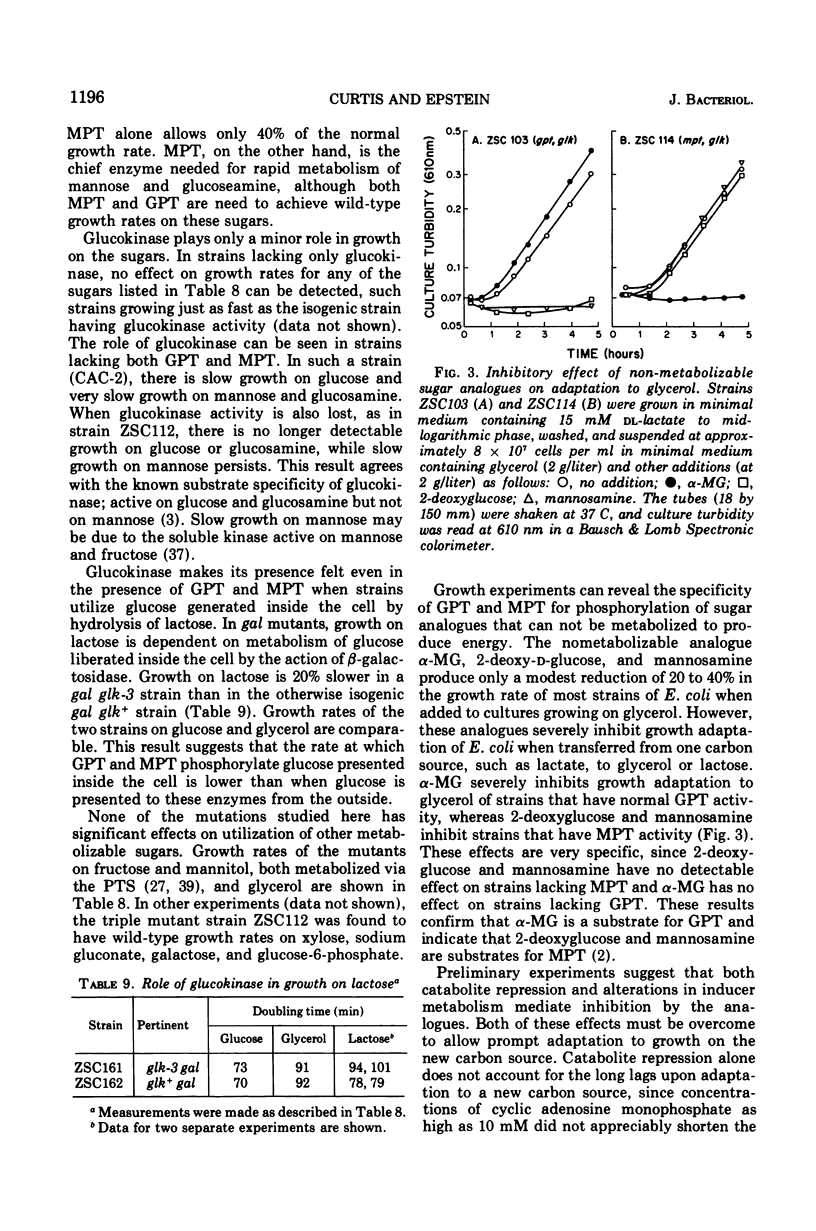
Genetic studies show that Escherichia coli has three enzymes capable of phosphorylating glucose: soluble adenosine 5'-triphosphate-dependent glucokinase, which plays only a minor role in glucose metabolism; an enzyme II, called glucosephosphotransferase, with high specificity for the D-glucose configuration; and another enzyme II, called mannosephosphotransferase, with broader specificity. The former enzyme II is active on glucose and methyl-alpha-glucopyranoside, whereas the latter is active on D-glucose, D-mannose, 2-deoxy-D-glucose, D-glucosamine, and D-mannosamine. Mutations leading to loss of glucosephosphotransferase activity and designated by the symbol gpt are between the purB and pyrC markers in a locus previously called cat. The locus of mutations to loss of mannosephosphotransferase, mpt, is between the eda and fadD genes. Mutations to loss of glucokinase, glk, are between the ptsI and dsd genes.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J., Epstein W. Phosphotransferase-system enzymes as chemoreceptors for certain sugars in Escherichia coli chemotaxis. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2895–2899. doi: 10.1073/pnas.71.7.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURSTEIN C., COHN M., KEPES A., MONOD J. R OLE DU LACTOSE ET DE SES PRODUITS M'ETABOLIQUES DANS L'INDUCTION DE L'OP'ERON LACTOSE CHEZ ESCHERICHIA COLI. Biochim Biophys Acta. 1965 Apr 19;95:634–639. [PubMed] [Google Scholar]
- Dietz G. W., Heppel L. A. Studies on the uptake of hexose phosphates. I. 2-Deoxyglucose and 2-deoxyglucose 6-phosphate. J Biol Chem. 1971 May 10;246(9):2881–2884. [PubMed] [Google Scholar]
- Epstein W., Davies M. Potassium-dependant mutants of Escherichia coli K-12. J Bacteriol. 1970 Mar;101(3):836–843. doi: 10.1128/jb.101.3.836-843.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Jewett S., Fox C. F. Isolation and mapping of phosphotransferase mutants in Escherichia coli. J Bacteriol. 1970 Nov;104(2):793–797. doi: 10.1128/jb.104.2.793-797.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAENKEL D. G., FALCOZ-KELLY F., HORECKER B. L. THE UTILIZATION OF GLUCOSE 6-PHOSPHATE BY GLUCOKINASELESS AND WILD-TYPE STRAINS OF ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1964 Nov;52:1207–1213. doi: 10.1073/pnas.52.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAENKEL D. G., HORECKER B. L. PATHWAYS OF D-GLUCOSE METABOLISM IN SALMONELLA TYPHINMURIUM. A STUDY OF A MUTANT LACKING PHOSPHOGLUCOSE ISOMERASE. J Biol Chem. 1964 Sep;239:2765–2771. [PubMed] [Google Scholar]
- Ferenci T., Kornberg H. L. The role of phosphotransferase-mediated syntheses of fructose 1-phosphate and fructose 6-phosphate in the growth of Escherichia coli on fructose. Proc R Soc Lond B Biol Sci. 1974 Sep 17;187(1087):105–119. doi: 10.1098/rspb.1974.0065. [DOI] [PubMed] [Google Scholar]
- Fox C. F., Wilson G. The role of a phosphoenolpyruvate-dependent kinase system in beta-glucoside catabolism in Escherichia coli. Proc Natl Acad Sci U S A. 1968 Mar;59(3):988–995. doi: 10.1073/pnas.59.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M. M., Hicks M. L., Gellert M. Genetics and function of DNA ligase in Escherichia coli. J Mol Biol. 1973 Jul 15;77(4):531–547. doi: 10.1016/0022-2836(73)90221-0. [DOI] [PubMed] [Google Scholar]
- HAGIHIRA H., WILSON T. H., LIN E. C. STUDIES ON THE GLUCOSE-TRANSPORT SYSTEM IN ESCHERICHIA COLI WITH ALPHA-METHYLGLUCOSIDE AS SUBSTRATE. Biochim Biophys Acta. 1963 Nov 15;78:505–515. doi: 10.1016/0006-3002(63)90912-0. [DOI] [PubMed] [Google Scholar]
- HOFFEE P., ENGLESBERG E., LAMY F. THE GLUCOSE PERMEASE SYSTEM IN BACTERIA. Biochim Biophys Acta. 1964 Mar 30;79:337–350. [PubMed] [Google Scholar]
- Hayashi S. I., Lin E. C. Product induction of glycerol kinase in Escherichia coli. J Mol Biol. 1965 Dec;14(2):515–521. doi: 10.1016/s0022-2836(65)80200-5. [DOI] [PubMed] [Google Scholar]
- Helling R. B., Adams B. S. Nalidixic acid-resistant auxotrophs of Escherichia coli. J Bacteriol. 1970 Nov;104(2):1027–1029. doi: 10.1128/jb.104.2.1027-1029.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe A., Bourgeois S. lac Repressor-operator interaction. VI. The natural inducer of the lac operon. J Mol Biol. 1972 Aug 28;69(3):397–408. doi: 10.1016/0022-2836(72)90253-7. [DOI] [PubMed] [Google Scholar]
- KESSLER D. P., RICKENBERG H. V. A NEW METHOD FOR THE SELECTION OF MUTANTS OF ESCHERICHIA COLI FORMING BETA-GALACTOSIDASE CONSTITUTIVELY. Biochim Biophys Acta. 1964 Sep 4;90:609–610. doi: 10.1016/0304-4165(64)90241-7. [DOI] [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R. Transport. Annu Rev Biochem. 1970;39:561–598. doi: 10.1146/annurev.bi.39.070170.003021. [DOI] [PubMed] [Google Scholar]
- Konrad E. B., Modrich P., Lehman I. R. Genetic and enzymatic characterization of a conditional lethal mutant of Escherichia coli K12 with a temperature-sensitive DNA ligase. J Mol Biol. 1973 Jul 15;77(4):519–529. doi: 10.1016/0022-2836(73)90220-9. [DOI] [PubMed] [Google Scholar]
- Kornberg H. L., Smith J. Genetic control of glucose uptake by Escherichia coli. FEBS Lett. 1972 Feb 15;20(3):270–272. doi: 10.1016/0014-5793(72)80084-x. [DOI] [PubMed] [Google Scholar]
- Kundig W., Kundig F. D., Anderson B., Roseman S. Restoration of active transport of glycosides in Escherichia coli by a component of a phosphotransferase system. J Biol Chem. 1966 Jul 10;241(13):3243–3246. [PubMed] [Google Scholar]
- Kundig W., Roseman S. Sugar transport. I. Isolation of a phosphotransferase system from Escherichia coli. J Biol Chem. 1971 Mar 10;246(5):1393–1406. [PubMed] [Google Scholar]
- Kundig W., Roseman S. Sugar transport. II. Characterization of constitutive membrane-bound enzymes II of the Escherichia coli phosphotransferase system. J Biol Chem. 1971 Mar 10;246(5):1407–1418. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lengeler J., Lin E. C. Reversal of the mannitol-sorbitol diauxie in Escherichia coli. J Bacteriol. 1972 Nov;112(2):840–848. doi: 10.1128/jb.112.2.840-848.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis W. F., Jr, Magasanik B. Genetic control of catabolite repression of the lac operon in Escherichia coli. Biochem Biophys Res Commun. 1965 Jul 12;20(2):230–234. doi: 10.1016/0006-291x(65)90351-7. [DOI] [PubMed] [Google Scholar]
- Overath P., Pauli G., Schairer H. U. Fatty acid degradation in Escherichia coli. An inducible acyl-CoA synthetase, the mapping of old-mutations, and the isolation of regulatory mutants. Eur J Biochem. 1969 Feb;7(4):559–574. [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- PLANAS J., DE CASTRO S. [The transport of blood iron in some mammals]. Rev Esp Fisiol. 1960 Jun;16:121–128. [PubMed] [Google Scholar]
- Pastan I., Perlman R. Cyclic adenosine monophosphate in bacteria. Science. 1970 Jul 24;169(3943):339–344. doi: 10.1126/science.169.3943.339. [DOI] [PubMed] [Google Scholar]
- Pouyssegur J. M. Localisation génétique de mutations 2-céto-3-désoxy-6-P-gluconate aldolase négatives chez E. coli K 12. Mol Gen Genet. 1971;113(1):31–42. [PubMed] [Google Scholar]
- SHERMAN J. R. Rapid enzyme assay technique utilizing radioactive substrate, ion-exchange paper, and liquid scintillation counting. Anal Biochem. 1963 Jun;5:548–554. doi: 10.1016/0003-2697(63)90075-7. [DOI] [PubMed] [Google Scholar]
- Schaefler S. Inducible system for the utilization of beta-glucosides in Escherichia coli. I. Active transport and utilization of beta-glucosides. J Bacteriol. 1967 Jan;93(1):254–263. doi: 10.1128/jb.93.1.254-263.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastián J., Asensio C. Identification of mannokinase in Escherichia coli. Biochem Biophys Res Commun. 1967 Jul 21;28(2):197–202. doi: 10.1016/0006-291x(67)90429-9. [DOI] [PubMed] [Google Scholar]
- Solomon E., Lin E. C. Mutations affecting the dissimilation of mannitol by Escherichia coli K-12. J Bacteriol. 1972 Aug;111(2):566–574. doi: 10.1128/jb.111.2.566-574.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Fraenkel D. G., Lin E. C. The enzymatic lesion of strain MM-6, a pleiotropic carbohydrate-negative mutant of Escherichia coli. Biochem Biophys Res Commun. 1967 Apr 7;27(1):63–67. doi: 10.1016/s0006-291x(67)80040-8. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler B., Wishnow R., Loomis W. F., Jr, Magasanik B. Catabolite repression gene of Escherichia coli. J Bacteriol. 1969 Nov;100(2):809–816. doi: 10.1128/jb.100.2.809-816.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaig N., Lin E. C. Feedback inhibition of glycerol kinase, a catabolic enzyme in Escherichia coli. Science. 1966 Aug 12;153(3737):755–757. doi: 10.1126/science.153.3737.755. [DOI] [PubMed] [Google Scholar]



