Abstract
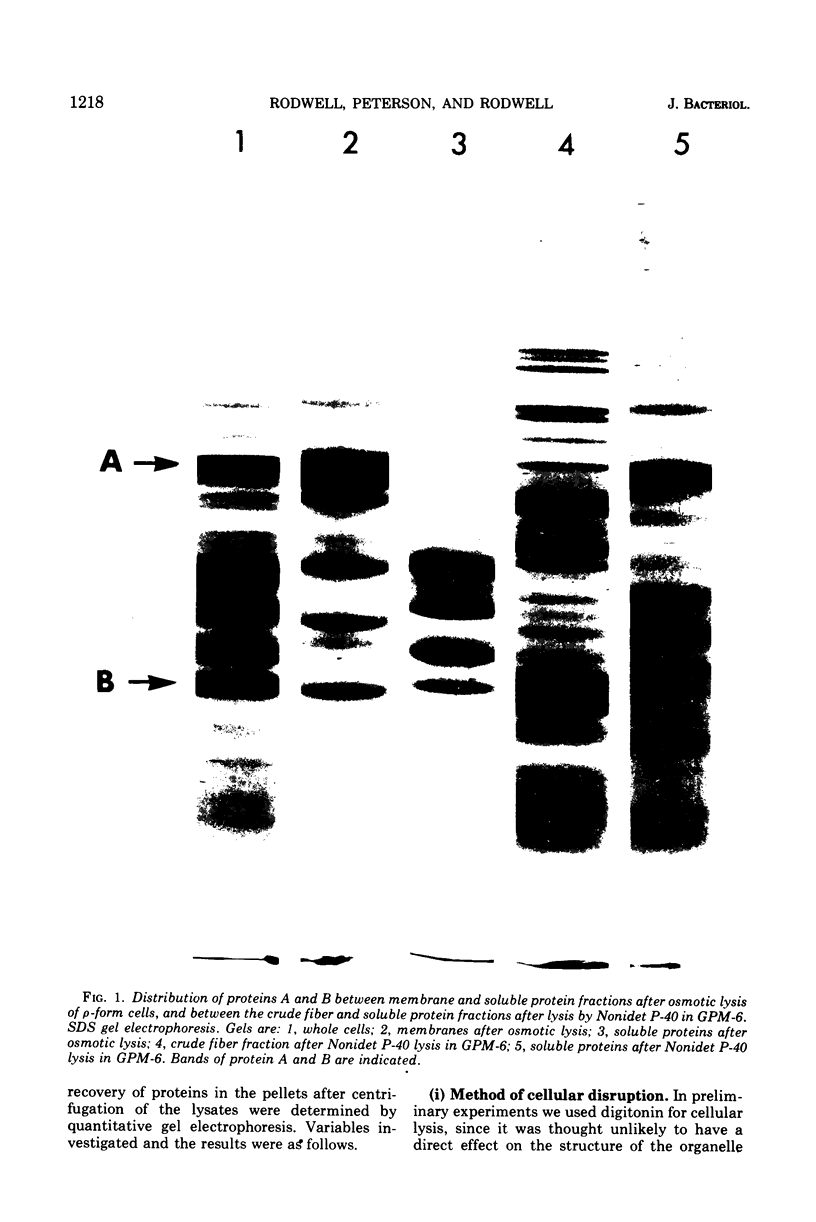
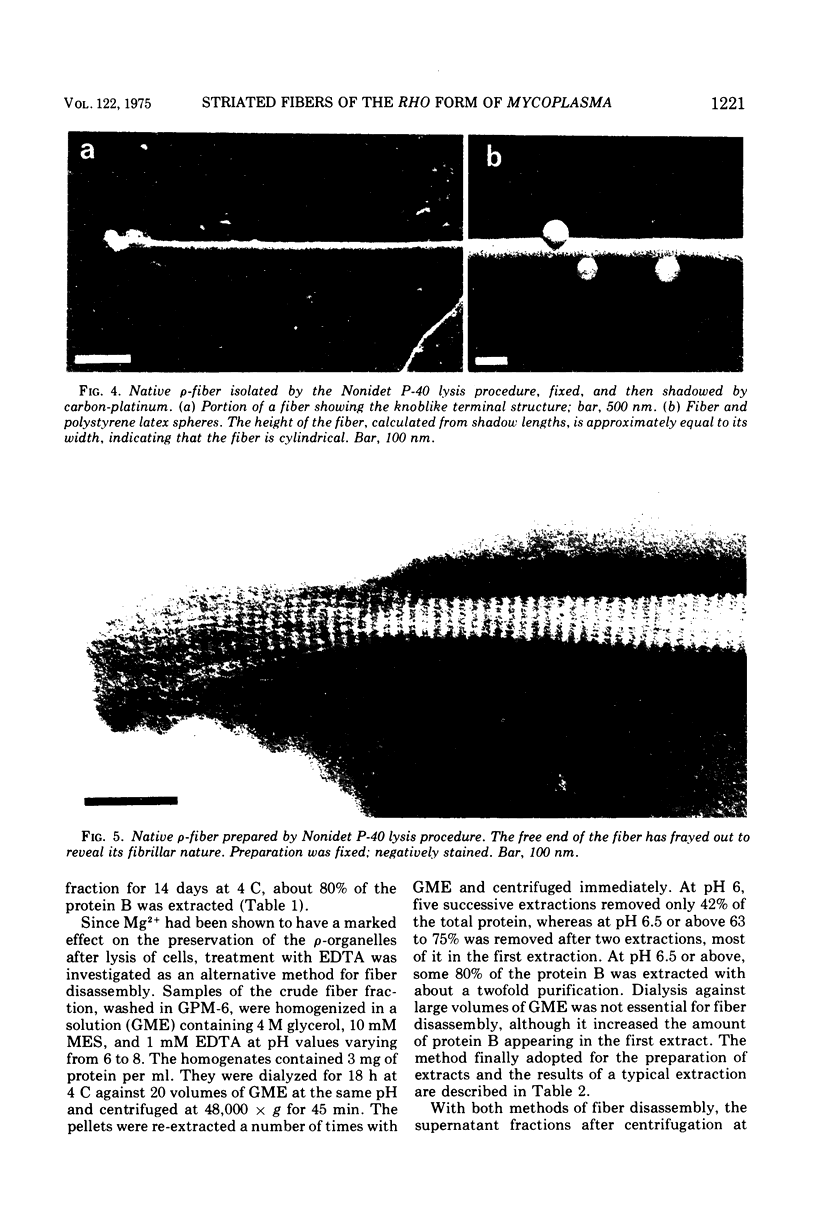
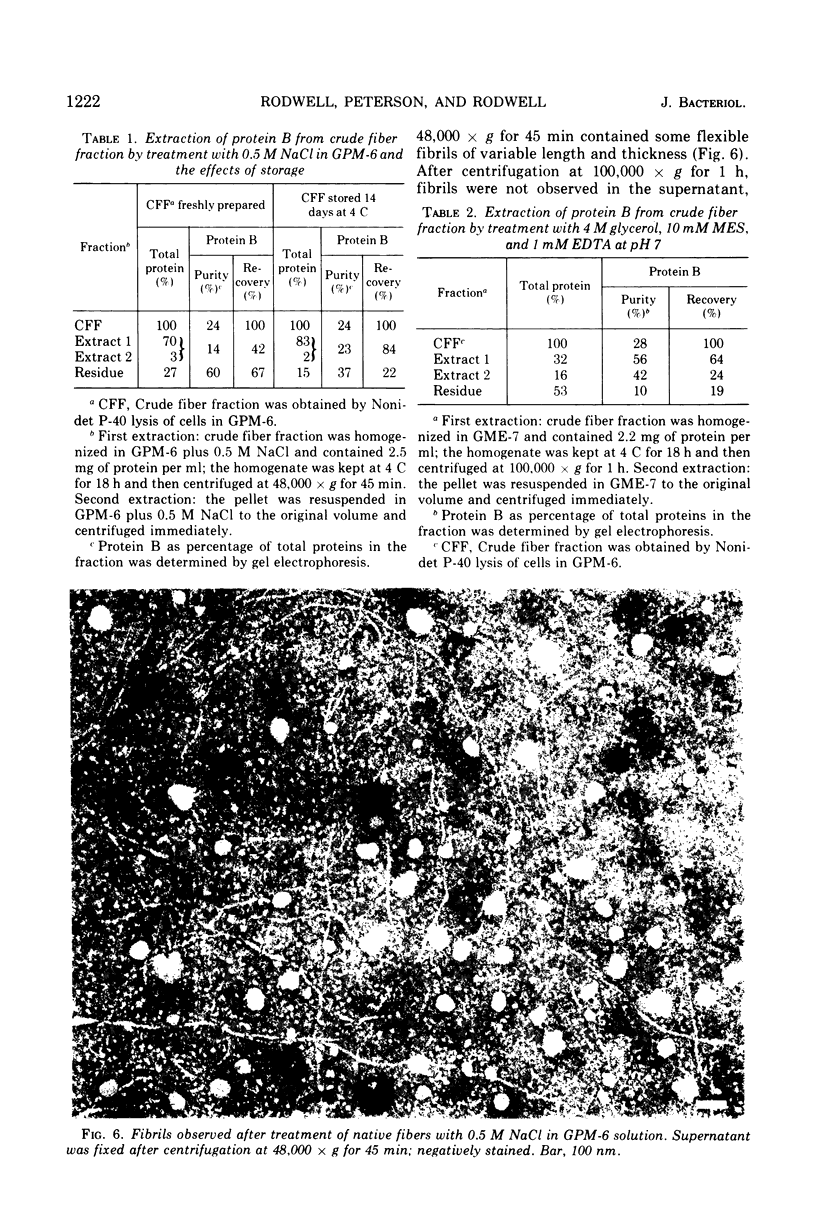
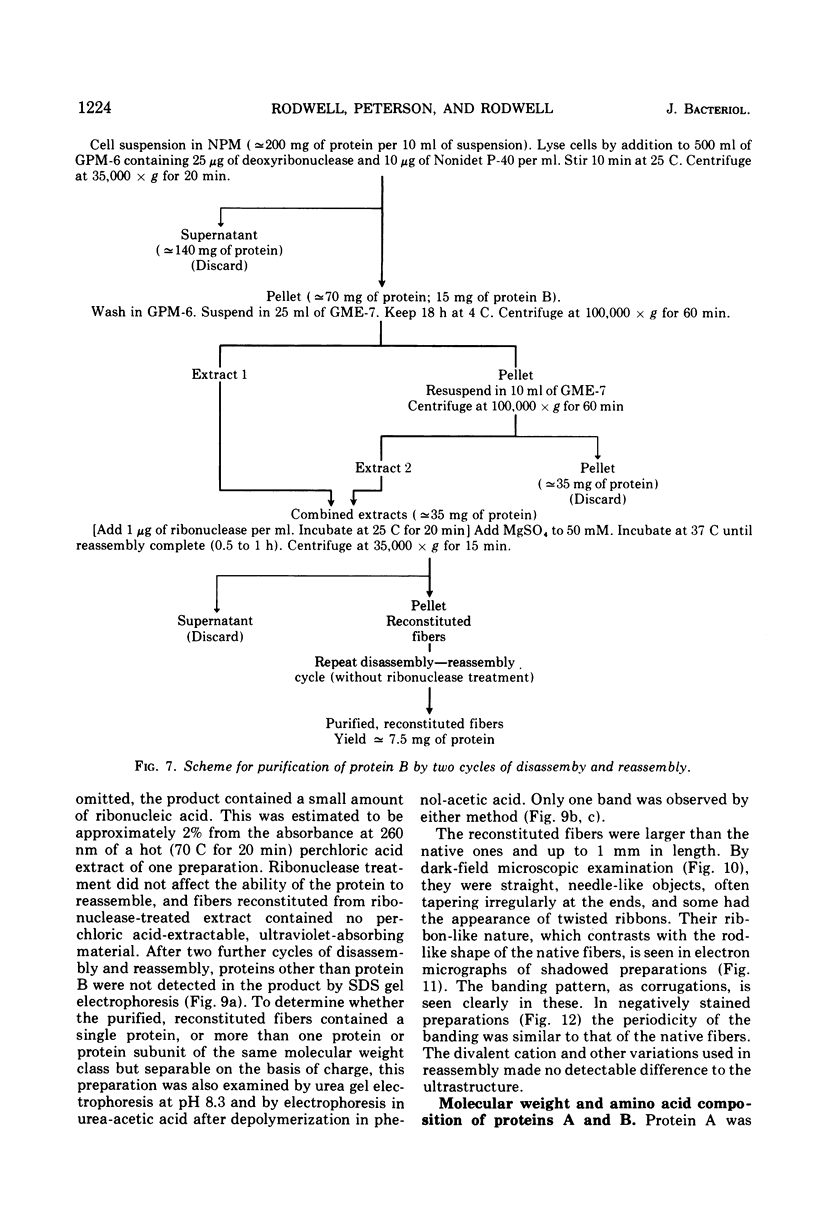
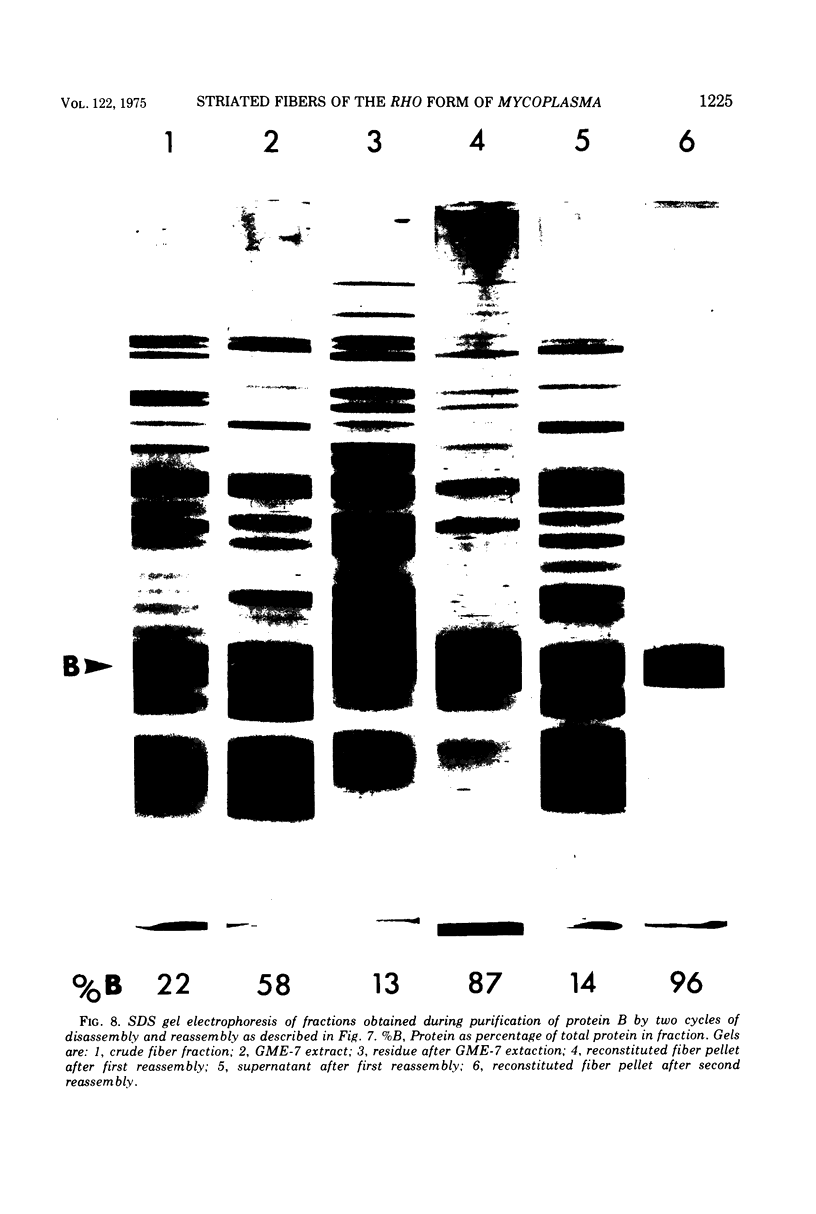
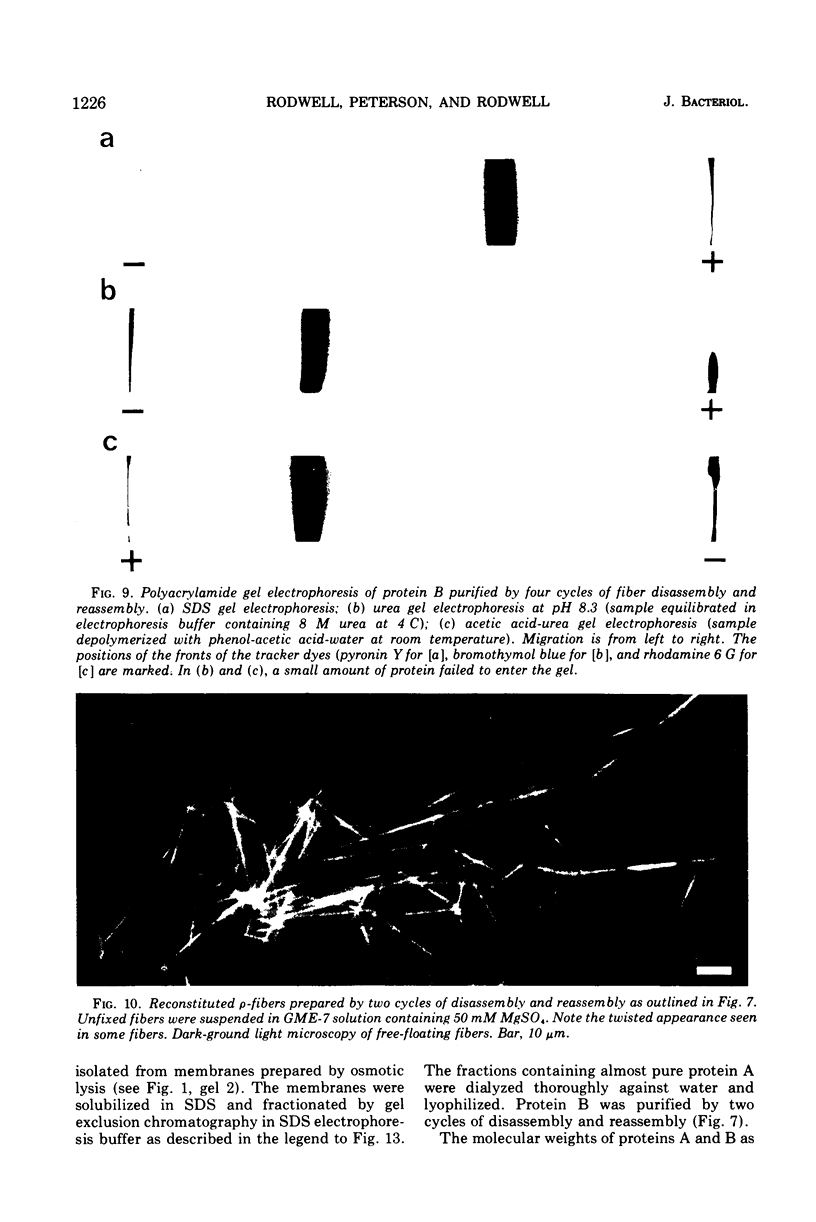
The rho-form of Mycoplasma contains a striated, axial fiber and associated terminal structure. The presence of this organelle was correlated with the synthesis of two proteins, A and B, of molecular weights of approximately 85,000 and 26,000, respectively, each accounting for about 10% of the total cell protein. Their amino acid compositions showed them to have distinct polypeptide chains. After osmotic lysis of rho-form cells the organelles disappeared; protein A accompanied the membrane fraction, whereas protein B was partly released in soluble form. After lysis by Nonidet P-40 in a medium composed of 4 M glycerol, 50 mM phosphate, and 10 mM MgSO4 at pH 6 (GPM-6), the organelles were preserved and released with ultrastructure unchanged. Protein A was recovered in the soluble fraction and protein B in the particulate (crude fiber) fraction. Treatment of the crude fiber fraction with 0.5 M NaCl in GPM-6 or with a solution containing 4 M glycerol, 10 mM morpholinoethanesulfonate, and 1 mM ethylenediaminetetraacetate at pH 7.0 caused the fibers to disassemble into subunits. By subsequent changes in the ionic conditions and temperature it was possible to cause the subunits to reassemble into ordered aggregates having the same ultrastructure as the native rho-fibers. The optimum temperature for reassembly in the presence of 4 M glycerol was 37 C, the optimum pH was 6.5 to 7.0, and the presence of Mg-2+, replaceable by Ca-2+, SR-2+, or Ba-2+, was essential. Protein B was the only protein detected in the purified, reconsituted fibers.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- KANE R. E. THE MITOTIC APPARATUS. PHYSICAL-CHEMICAL FACTORS CONTROLLING STABILITY. J Cell Biol. 1965 Apr;25:SUPPL–SUPPL:144. doi: 10.1083/jcb.25.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffer M. A., Stevens C. L. Structure of the tobacco mosaic virus particle; polymerization of tobacco mosaic virus protein. Adv Virus Res. 1968;13:1–63. doi: 10.1016/s0065-3527(08)60250-x. [DOI] [PubMed] [Google Scholar]
- Peterson J. E., Rodwell A. W., Rodwell E. S. Occurrence and ultrastructure of a variant (rho) form of Mycoplasma. J Bacteriol. 1973 Jul;115(1):411–425. doi: 10.1128/jb.115.1.411-425.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K., MacLennan D. H., Tzagoloff A., Stoner C. D. Studies on the electron transfer system. LXVII. Polyacrylamide gel electrophoresis of the mitochondrial electron transfer complexes. Arch Biochem Biophys. 1966 Apr;114(1):223–230. doi: 10.1016/0003-9861(66)90324-9. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weisenberg R. C. Microtubule formation in vitro in solutions containing low calcium concentrations. Science. 1972 Sep 22;177(4054):1104–1105. doi: 10.1126/science.177.4054.1104. [DOI] [PubMed] [Google Scholar]