Abstract
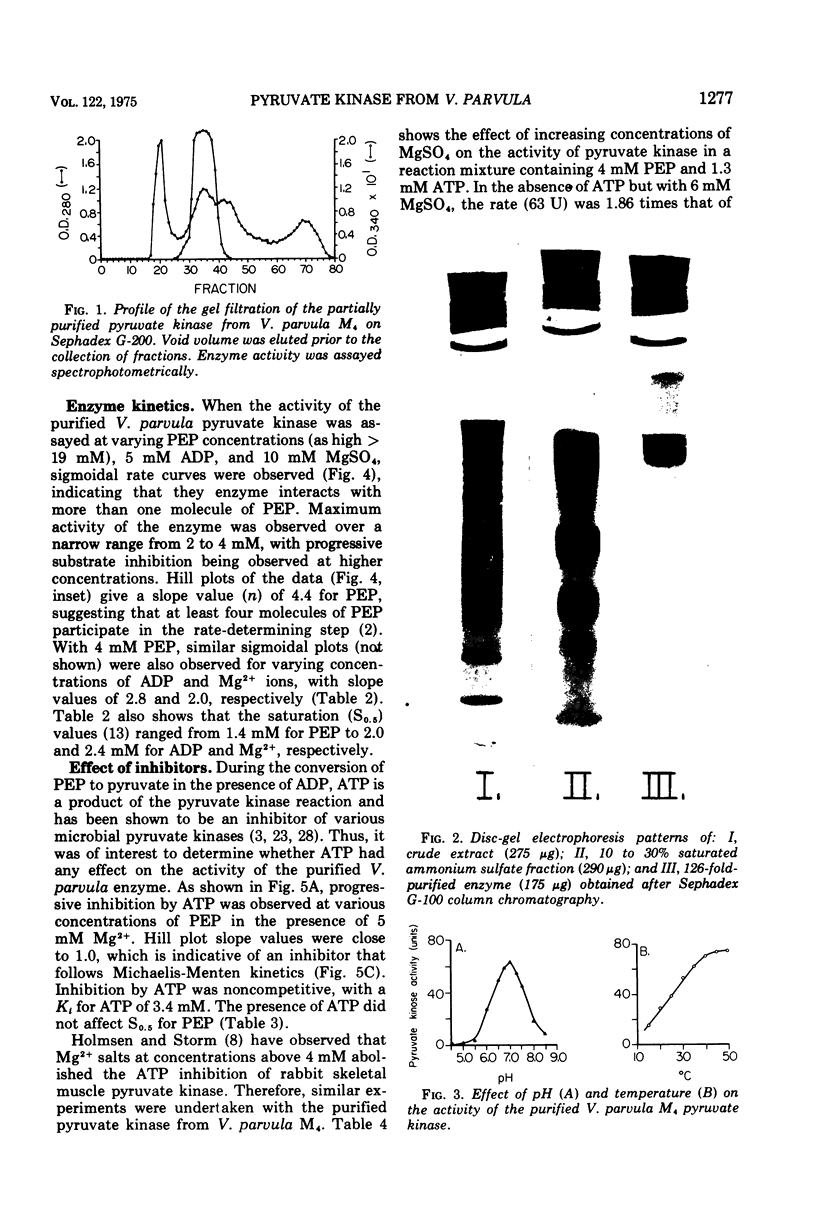
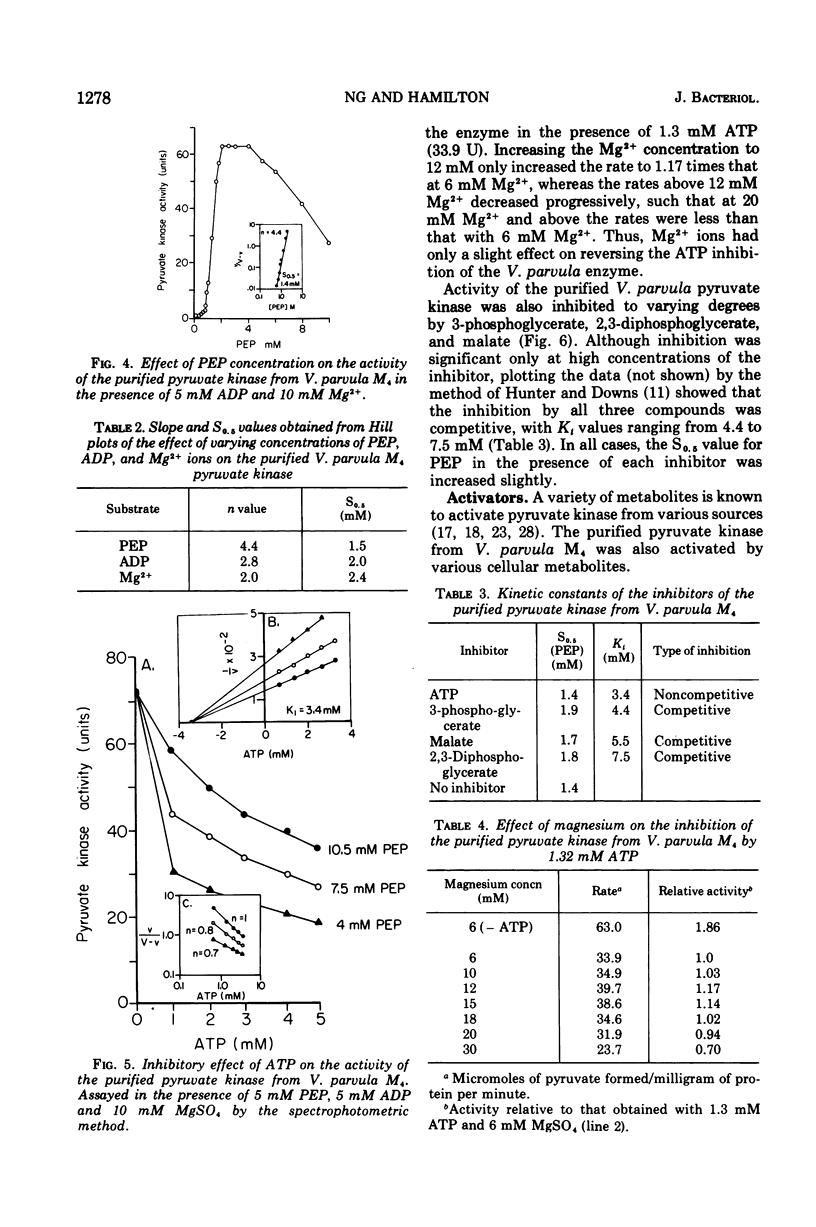
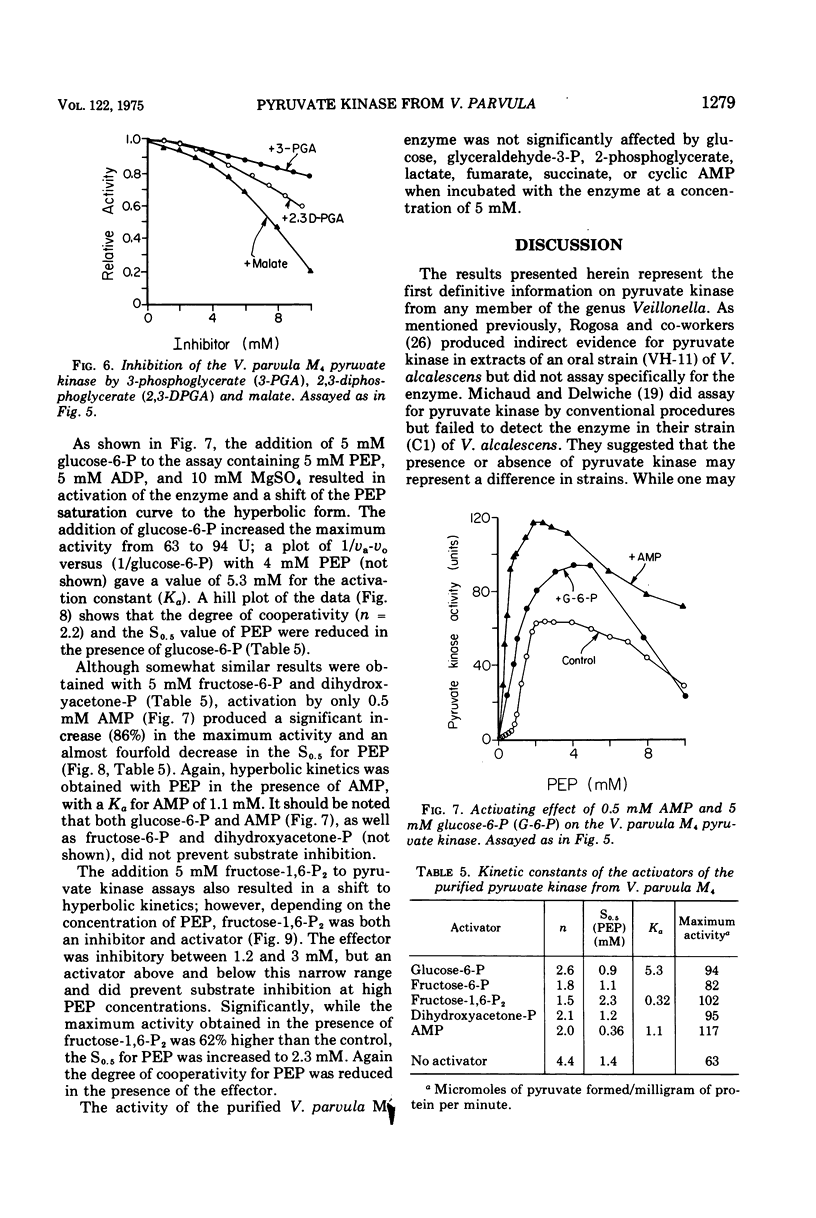
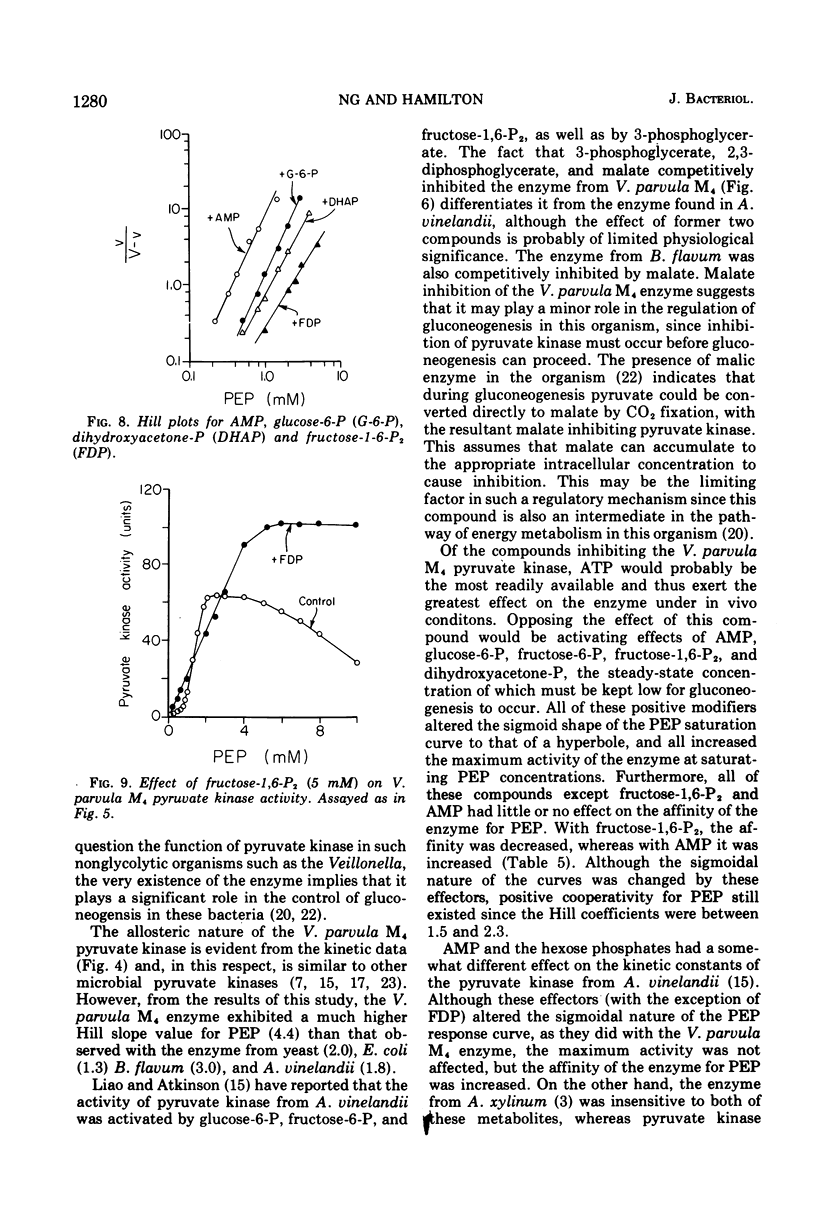
The nonglycolytic, anaerobic organism Veillonella parvula M4 has been shown to contain an active pyruvate kinase. The enzyme was purified 126-fold and was shown by disc-gel electrophoresis to contain only two faint contaminating bands. The purified enzyme had a pH optimum of 7.0 in the forward direction and exhibited sigmoidal kinetics at varying concentrations o-f phosphoenol pyruvate (PEP), adenosine 5'-monophosphate (AMP), and Mg-2+ ions with S0.5 values of 1.5, 2.0, and 2.4 mM, respectively. Substrate inhibition was observed above 4 m PEP. Hill plots gave slope values (n) of 4.4 (PEP), 2.8 (adenosine 5'-diphosphate), and 2.0 (Mg-2+), indicating a high degree of cooperativity. The enzyme was inhibited non-competitively by adenosine 5'-triphosphate (Ki = 3.4 mM), and this inhibition was only slightly affected by increasing concentration of Mg-2+ ions to 30 mM. Competitive inhibition was observed with 3-phosphoglycerate, malate, and 2,3-diphosphoglycerate but only at higher inhibitor concentrations. The enzyme was activated by glucose-6-phosphate (P), fructose-6-P, fructose-1,6-diphosphate (P2), dihydroxyacetone-P, and AMP; the Hill coefficients were 2.2, 1.8, 1.5, 2.1, and 2.0, respectively. The presence of each these metabolites caused substrate velocity curves to change from sigmoidal to hyperbolic curves, and each was accompanied by an increase in the maximum activity, e.g., AMP greater than fructose-1,6-P2 greater than dihydroxyacetone-P greater than glucose-6-P greater than fructose-6-P. The activation constants for fructose-1,6-P2, AMP, and glucose-6-P were 0.3, 1.1, and 5.3 mM, respectively. The effect of 5 mM fructose-1,6-P2 was significantly different from the other compounds in that this metabolite was inhibitory between 1.2 and 3 mM PEP. Above this concentration, fructose-1,6-P2 activated the enzyme and abolished substrate inhibition by PEP. The enzyme was not affected by glucose, glyceraldehyde-3-P, 2-phosphoglycerate, lactate, malate, fumerate, succinate, and cyclic AMP. The results suggest that the pyruvate kinase from V. parvula M4 plays a central role in the control of gluconeogenesis in this organism by regulating the concentration of PEP.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATKINSON D. E., HATHAWAY J. A., SMITH E. C. KINETICS OF REGULATORY ENZYMES. KINETIC ORDER OF THE YEAST DIPHOSPHOPYRIDINE NUCLEOTIDE ISOCITRATE DEHYDROGENASE REACTION AND A MODEL FOR THE REACTION. J Biol Chem. 1965 Jun;240:2682–2690. [PubMed] [Google Scholar]
- Atkinson D. E. Regulation of enzyme function. Annu Rev Microbiol. 1969;23:47–68. doi: 10.1146/annurev.mi.23.100169.000403. [DOI] [PubMed] [Google Scholar]
- Benziman M. Factors afecting the activity of pyruvate kinase of Acetobacter xylinum. Biochem J. 1969 May;112(5):631–636. doi: 10.1042/bj1120631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Foubert E. L., Douglas H. C. Studies on the Anaerobic Micrococci: II. The Fermentation of Lactate by Micrococcus lactilyticus. J Bacteriol. 1948 Jul;56(1):35–36. [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R., Burris R. H., Wilson P. W. Pyruvate metabolism by a nitrogen-fixing bacterium. Biochem J. 1965 Aug;96(2):383–389. doi: 10.1042/bj0960383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess B., Haeckel R., Brand K. FDP-activation of yeast pyruvate kinase. Biochem Biophys Res Commun. 1966 Sep 22;24(6):824–831. doi: 10.1016/0006-291x(66)90322-6. [DOI] [PubMed] [Google Scholar]
- Holmsen H., Storm E. The adenosine triphosphate inhibition of the pyruvate kinase reaction and its dependence on the total magnesium ion concentration. Biochem J. 1969 Apr;112(3):303–316. doi: 10.1042/bj1120303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsley J. R., Suelter C. H. Yeast pyruvate kinase. I. Purification and some chemical properties. J Biol Chem. 1969 Sep 25;244(18):4815–4818. [PubMed] [Google Scholar]
- Hunsley J. R., Suelter C. H. Yeast pyruvate kinase. II. Kinetic properties. J Biol Chem. 1969 Sep 25;244(18):4819–4822. [PubMed] [Google Scholar]
- JOHNS A. T. The mechanism of propionic acid formation by Veillonella gazogenes. J Gen Microbiol. 1951 May;5(2):326–336. doi: 10.1099/00221287-5-2-326. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liao C. L., Atkinson D. E. Regulation at the phosphoenolpyruvate branchpoint in Azotobacter vinelandii: pyruvate kinase. J Bacteriol. 1971 Apr;106(1):37–44. doi: 10.1128/jb.106.1.37-44.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeba P., Sanwal B. D. The regulation of pyruvate kinase of Escherichia coli by fructose diphosphate and adenylic acid. J Biol Chem. 1968 Jan 25;243(2):448–450. [PubMed] [Google Scholar]
- Malcovati M., Kornberg H. L. Two types of pyruvate kinase in Escherichia coli K12. Biochim Biophys Acta. 1969 Apr 22;178(2):420–423. doi: 10.1016/0005-2744(69)90417-3. [DOI] [PubMed] [Google Scholar]
- Michaud R. N., Delwiche E. A. Multiple impairment of glycolysis in Veillonella alcalescens. J Bacteriol. 1970 Jan;101(1):138–140. doi: 10.1128/jb.101.1.138-140.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng S. K., Hamilton I. R. Carbon dioxide fixation by Veillonella parvula M 4 and its relation to propionic acid formation. Can J Microbiol. 1973 Jun;19(6):715–723. doi: 10.1139/m73-116. [DOI] [PubMed] [Google Scholar]
- Ng S. K., Hamilton I. R. Gluconeogenesis by Veillonella parvula M4: evidence for the indirect conversion of pyruvate to P-enolpyruvate. Can J Microbiol. 1974 Jan;20(1):19–28. doi: 10.1139/m74-004. [DOI] [PubMed] [Google Scholar]
- Ng S. K., Hamilton I. R. Lactate metabolism by Veillonella parvula. J Bacteriol. 1971 Mar;105(3):999–1005. doi: 10.1128/jb.105.3.999-1005.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H., Shiio I. Regulation of the TCA and glyoxylate cycles in Brevibacterium flavum. II. Regulation of phosphoenolpyruvate carboxylase and pyruvate kinase. J Biochem. 1969 Sep;66(3):297–311. doi: 10.1093/oxfordjournals.jbchem.a129148. [DOI] [PubMed] [Google Scholar]
- ROGOSA M. THE GENUS VEILLONELLA. I. GENERAL CULTURAL, ECOLOGICAL, AND BIOCHEMICAL CONSIDERATIONS. J Bacteriol. 1964 Jan;87:162–170. doi: 10.1128/jb.87.1.162-170.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogosa M., Krichevsky M. I., Bishop F. S. Truncated Glycolytic System in Veillonella. J Bacteriol. 1965 Jul;90(1):164–171. doi: 10.1128/jb.90.1.164-171.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogosa M. The Genus Veillonella IV. Serological Groupings, and Genus and Species Emendations. J Bacteriol. 1965 Sep;90(3):704–709. doi: 10.1128/jb.90.3.704-709.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen F. W., Bernlohr R. W. Pyruvate kinase of the spore-forming bacterium, Bacillus licheniformis. II. Kinetic properties. J Biol Chem. 1971 Mar 25;246(6):1746–1755. [PubMed] [Google Scholar]
- Waygood E. B., Sanwal B. D. The control of pyruvate kinases of Escherichia coli. I. Physicochemical and regulatory properties of the enzyme activated by fructose 1,6-diphosphate. J Biol Chem. 1974 Jan 10;249(1):265–274. [PubMed] [Google Scholar]




