Abstract
Two different sizes of circular covalently closed deoxyribonucleic acid plasmids have been identified in four independent clinical isolates of eisseria gonorrhoeae. All four strains contained a small plasmid with a molecular weight of 2.8 X 10-6 and two of the four stains also contained a large plasmid with a molecular weight of 24.5 X 10-6. The avirulent derivative of each of these four strains had the same plasmid complement as its virulent parent. There was no correlation between the presence of these plasmids and antibiotic resistance, piliation, and colony type associated with virulence, or ability to grow without seven specific amino acid supplements.
Full text
PDF


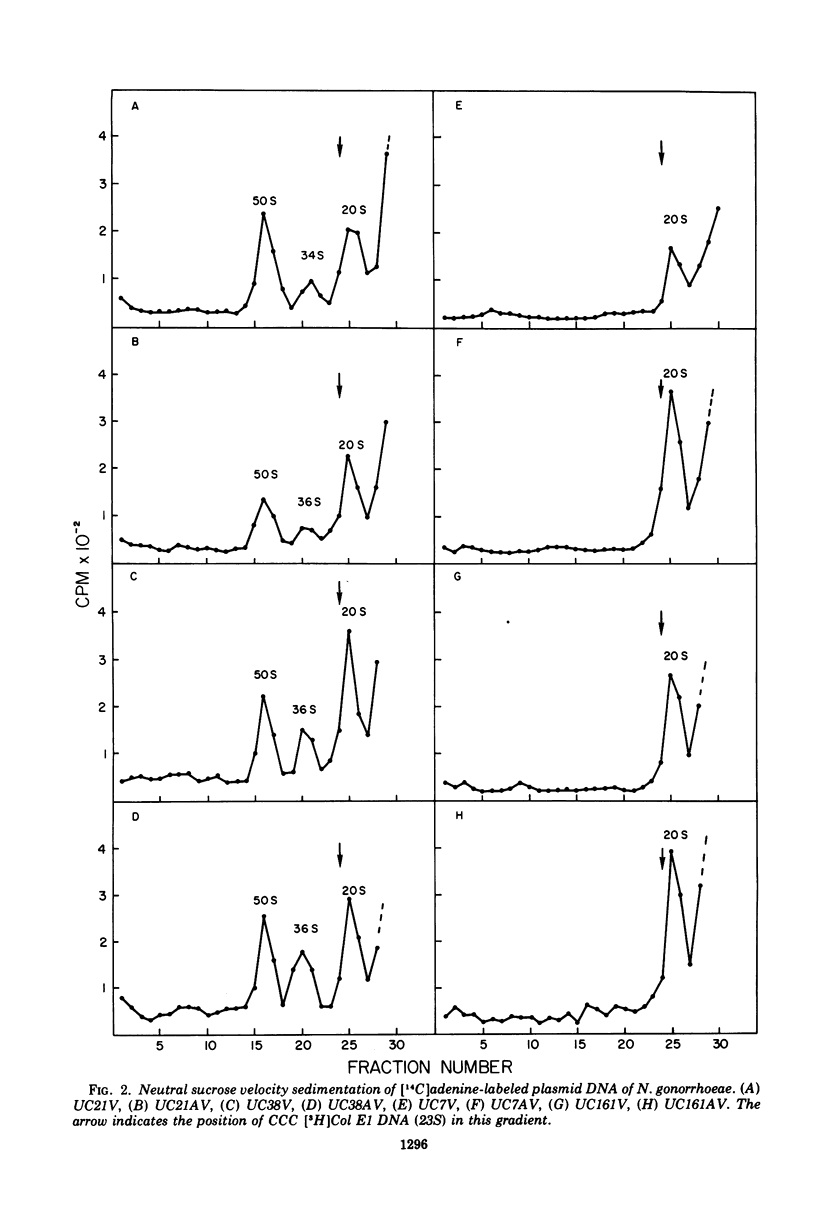




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amies C. R. Sensitivity of Neisseria gonorrhoeae to penicillin and other antibiotics. Studies carried out in Toronto during the period 1961 to 1968. Br J Vener Dis. 1969 Sep;45(3):216–222. doi: 10.1136/sti.45.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Characterization of multiple circular DNA forms of colicinogenic factor E-1 from Proteus mirabilis. Biochemistry. 1968 Oct;7(10):3513–3520. doi: 10.1021/bi00850a028. [DOI] [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Circular DNA forms of colicinogenic factors E1, E2 and E3 from Escherichia coli. J Mol Biol. 1968 Sep 14;36(2):185–194. doi: 10.1016/0022-2836(68)90374-4. [DOI] [PubMed] [Google Scholar]
- Buchanan T. M., Gotschlich E. C. Studies on gonococcus infection. 3. Correlation of gonococcal colony morphology with infectivity for the chick embryo. J Exp Med. 1973 Jan 1;137(1):196–200. doi: 10.1084/jem.137.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN M. L., MIDDLEBROOK G. Carbon dioxide-impermeable plastic bags for artificial cultivation of tubercle bacilli. Am Rev Respir Dis. 1963 Feb;87:292–293. doi: 10.1164/arrd.1963.87.2.292. [DOI] [PubMed] [Google Scholar]
- Catlin B. W. Genetic transformation of biosynthetically defective Neisseria gonorrhoeae clinical isolates. J Bacteriol. 1974 Oct;120(1):203–209. doi: 10.1128/jb.120.1.203-209.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes R. C. Molecular structure of bacterial plasmids. Bacteriol Rev. 1972 Sep;36(3):361–405. doi: 10.1128/br.36.3.361-405.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelkirk P. G., Schoenhard D. E. Physical evidence of a plasmid in Neisseria gonorrhoeae. J Infect Dis. 1973 Feb;127(2):197–200. doi: 10.1093/infdis/127.2.197. [DOI] [PubMed] [Google Scholar]
- Flynn J., Waitkins S. A. Survival of Neisseria gonorrhoeae in an artificial subcutaneous cavity of the mouse. Br J Vener Dis. 1973 Oct;49(5):432–434. doi: 10.1136/sti.49.5.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helinski D. R. Plasmid determined resistance to antibiotics: molecular properties of R factors. Annu Rev Microbiol. 1973;27:437–470. doi: 10.1146/annurev.mi.27.100173.002253. [DOI] [PubMed] [Google Scholar]
- Jephcott A. E., Reyn A., Birch-Andersen A. Neisseria gonorrhoeae 3. Demonstration of presumed appendages to cells from different colony types. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(3):437–439. doi: 10.1111/j.1699-0463.1971.tb00086.x. [DOI] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg D. S., Jr, Cohen I. R., Norins L. C., Schroeter A. L., Reising G. Neisseria gonorrhoeae. II. Colonial variation and pathogenicity during 35 months in vitro. J Bacteriol. 1968 Sep;96(3):596–605. doi: 10.1128/jb.96.3.596-605.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Scolea L. J., Jr, Young F. E. Development of a defined minimal medium for the growth of Neisseria gonorrhoeae. Appl Microbiol. 1974 Jul;28(1):70–76. doi: 10.1128/am.28.1.70-76.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D., Mitani M. Simplified quantitative electron microscopy of biopolymers. Biopolymers. 1970;9(3):373–379. doi: 10.1002/bip.1970.360090310. [DOI] [PubMed] [Google Scholar]
- Maier T. W., Beilstein H. R., Zubrzycki L. Multiple antibiotic resistance in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1974 Jul;6(1):22–28. doi: 10.1128/aac.6.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maness M. J., Sparling P. F. Multiple antibiotic resistance due to a single mutation in Neisseria gonorrhoeae. J Infect Dis. 1973 Sep;128(3):321–330. doi: 10.1093/infdis/128.3.321. [DOI] [PubMed] [Google Scholar]
- Mayer L. W., Holmes K. K., Falkow S. Characterization of plasmid deoxyribonucleic acid from Neisseria gonorrhoeae. Infect Immun. 1974 Oct;10(4):712–717. doi: 10.1128/iai.10.4.712-717.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P. Extrachromosomal inheritance in bacteria. Bacteriol Rev. 1969 Jun;33(2):210–263. doi: 10.1128/br.33.2.210-263.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Bisno A. L. Resistance of Neisseria gonorrhoeae to phagocytosis: relationship to colonial morphology and surface pili. J Infect Dis. 1974 Mar;129(3):310–316. doi: 10.1093/infdis/129.3.310. [DOI] [PubMed] [Google Scholar]
- Phillips I., Ridley M., Rimmer D., Lynn R. In-vitro activity of twelve antibacterial agents against Neisseria gonorrhoeae. Lancet. 1970 Feb 7;1(7641):263–265. doi: 10.1016/s0140-6736(70)90635-5. [DOI] [PubMed] [Google Scholar]
- Punsalang A. P., Jr, Sawyer W. D. Role of pili in the virulence of Neisseria gonorrhoeae. Infect Immun. 1973 Aug;8(2):255–263. doi: 10.1128/iai.8.2.255-263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYN A. Sensitivity of N. gonorrhoeae to antibiotics. Br J Vener Dis. 1961 Jun;37:145–157. doi: 10.1136/sti.37.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyn A., Bentzon M. W. Relationships between the sensitivities in vitro of Neisseria gonorrhoeae to spiramycin, penicillin, streptomycin, tetracycline, and erythromycin. Br J Vener Dis. 1969 Sep;45(3):223–227. doi: 10.1136/sti.45.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparling P. F. Genetic transformation of Neisseria gonorrhoeae to streptomycin resistance. J Bacteriol. 1966 Nov;92(5):1364–1371. doi: 10.1128/jb.92.5.1364-1371.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiffler P. W., Sweeney H. M., Cohen S. Absence of circular plasmid deoxyribonucleic acid attributable to a genetic determinant for methicillin resistance in Staphylococcus aureus. J Bacteriol. 1973 Nov;116(2):771–777. doi: 10.1128/jb.116.2.771-777.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiffler P. W., Sweeney H. M., Schneider M., Cohen S. Isolation and characterization of a kanamycin resistance plasmid from Staphylococcus aureus. Antimicrob Agents Chemother. 1974 Oct;6(4):516–520. doi: 10.1128/aac.6.4.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Kraus S. J., Gotschlich E. C. Studies on gonococcus infection. I. Pili and zones of adhesion: their relation to gonococcal growth patterns. J Exp Med. 1971 Oct 1;134(4):886–906. doi: 10.1084/jem.134.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J Exp Med. 1973 Mar 1;137(3):571–589. doi: 10.1084/jem.137.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongthai C., Sawyer W. D. Studies on the virulence of Neisseria gonorrhoeae. I. Relation of colonial morphology and resistance to phagocytosis by polymorphonuclear leukocytes. Infect Immun. 1973 Mar;7(3):373–379. doi: 10.1128/iai.7.3.373-379.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J. Physical and topological properties of circular DNA. J Gen Physiol. 1966 Jul;49(6):103–125. doi: 10.1085/jgp.49.6.103. [DOI] [PMC free article] [PubMed] [Google Scholar]




