Abstract
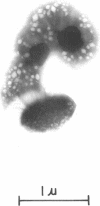
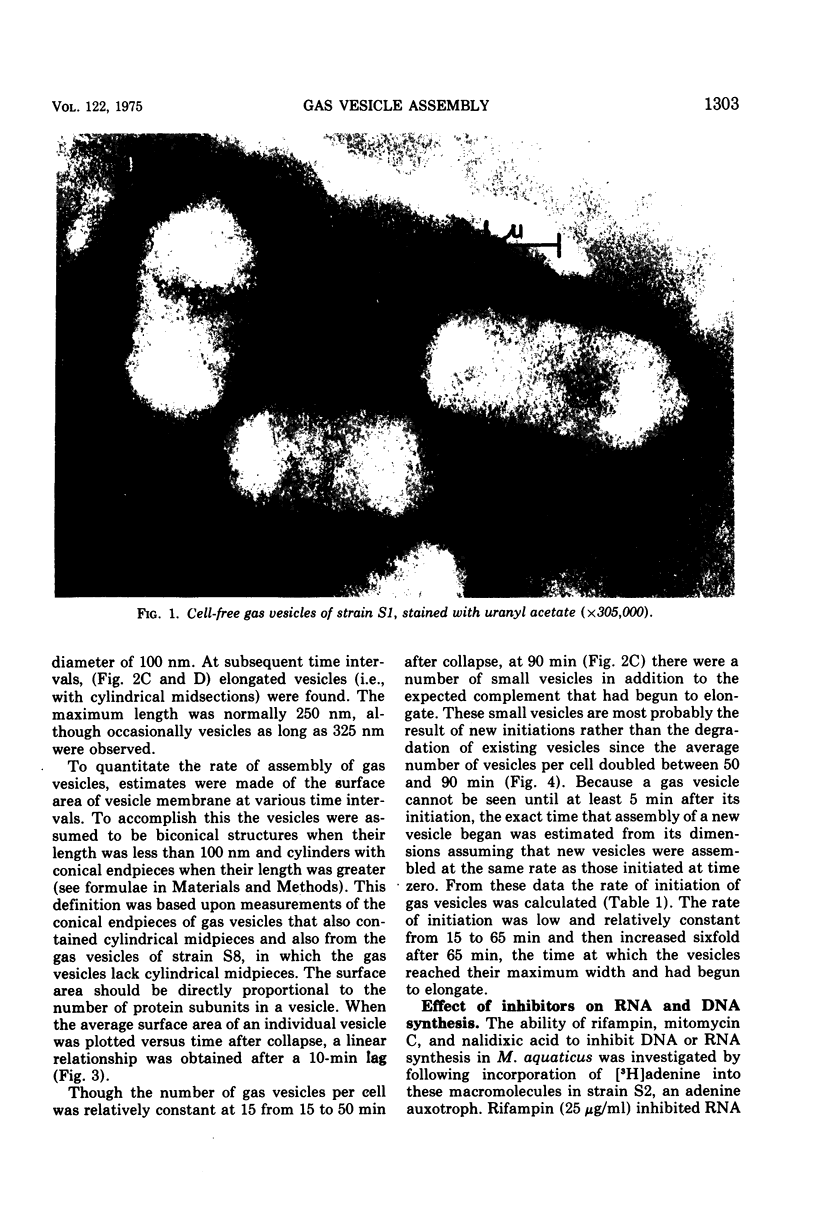
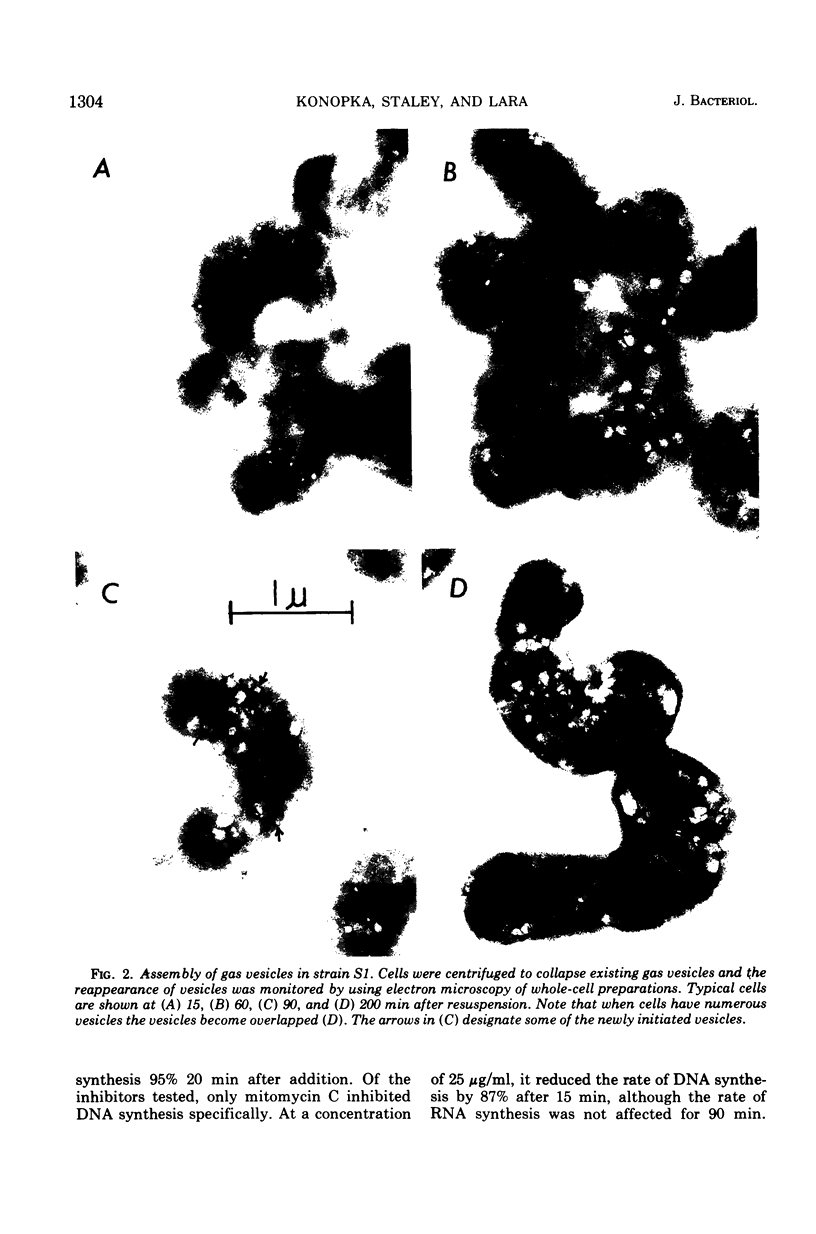
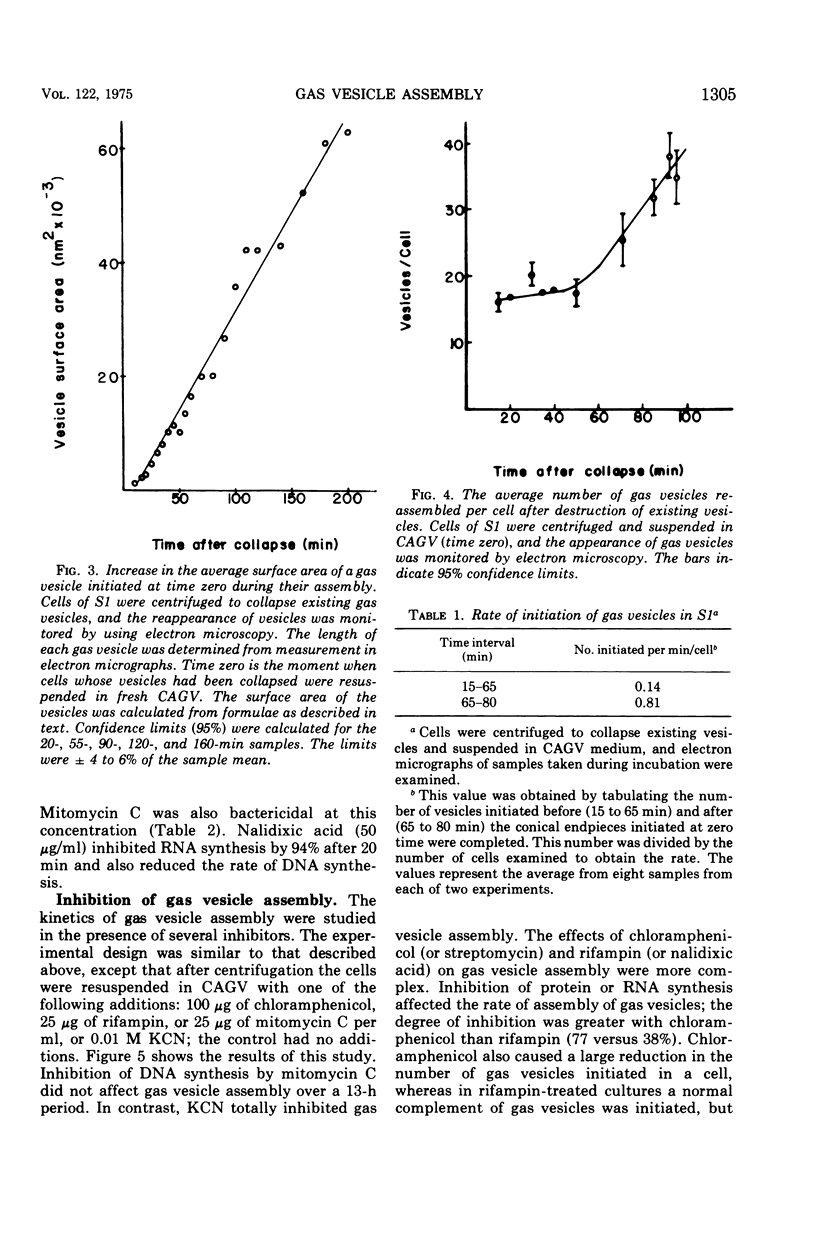
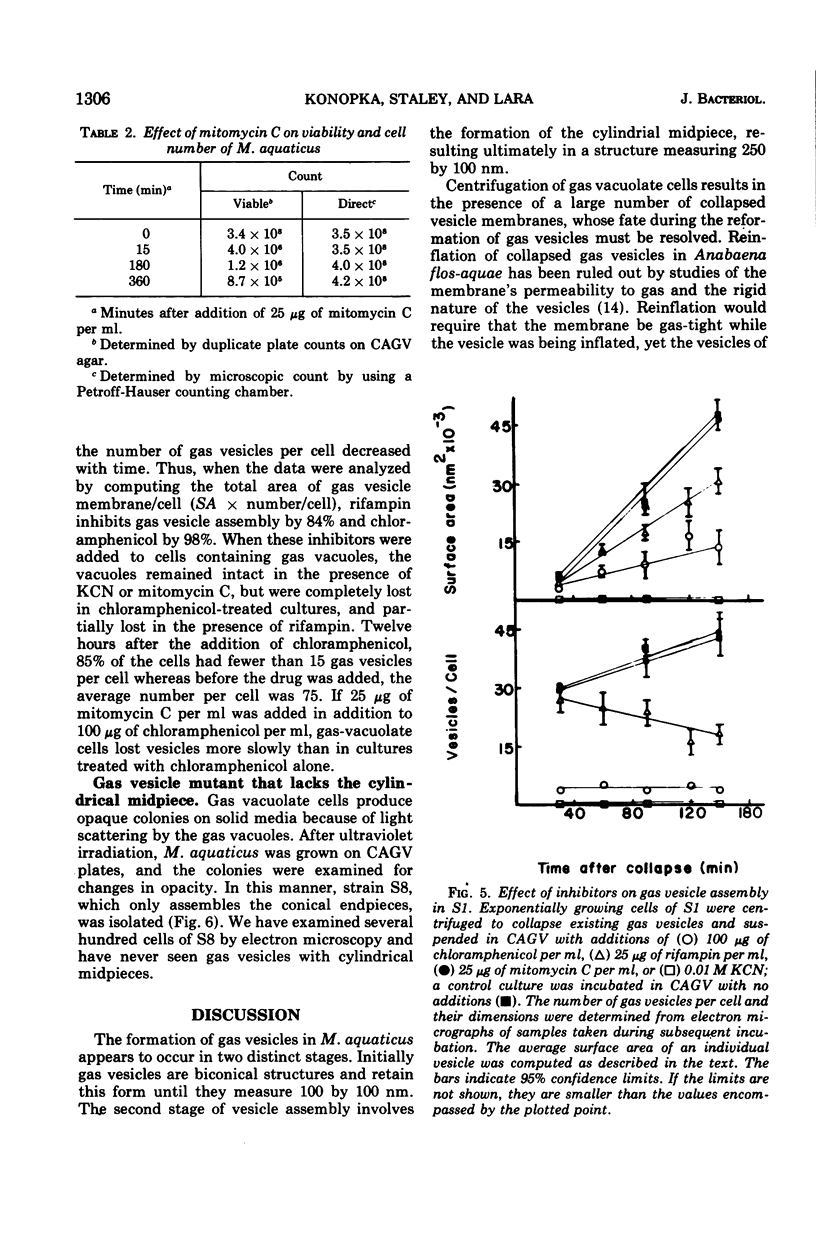
When observed in the electron microscope intact gas vesicles appeared as transparent areas in whole cells of Microcylus aquaticus, whereas vesicles collapsed by centrifugation were not discernible. Within 5 min of suspending cells containing collapsed vesicles in growth medium, small transparent vesicles were detected. By 15 min the average number of vesicles per cell was 15. This number remained relatively constant while the size of the vesicles increased until they attained their maximum diamtere of 100 nm. At this time the vesicles, interpreted as biconical structures, began to elongate presumably due to the synthesis of the cylindrical midsection. Closely correlated with the time at which vesicles began to elongate was the initiation of smaller vesicles which resulted in a doubling of the number of vesicles per cell by 90 min. This evidence coupled with the isolation of a mutant which assembles only the conical portions of the vesicle suggests that assembly occurs in two distinct stages subject to genetic mutation. Protein and ribonucleic acid synthesis, and presumably adenosine triphosphate formation, were required for gas vesicle assembly. In addition, inhibition of protein or ribonucleic acid synthesis resulted in a loss of extant gas vesicles. Over the time course of our study, deoxyribonucleic acid synthesis was not required for gas vesicle assembly or stability.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buckland B., Walsby A. E. A study of the strength and stability of gas vesicles isolated from a blue-green alga. Arch Mikrobiol. 1971;79(4):327–337. doi: 10.1007/BF00424908. [DOI] [PubMed] [Google Scholar]
- HOUWINK A. L. Flagella, gas vacuoles and cell-wall structure in Halobacterium halobium; an electron microscope study. J Gen Microbiol. 1956 Aug;15(1):146–150. doi: 10.1099/00221287-15-1-146. [DOI] [PubMed] [Google Scholar]
- Jones D. D., Jost M. Isolation and chemical characterization of gas-vacuole membranes from Microcystis aeruginosa Kuetz. emend. Elenkin. Arch Mikrobiol. 1970;70(1):43–64. doi: 10.1007/BF00691059. [DOI] [PubMed] [Google Scholar]
- Krantz M. J., Ballou C. E. Analysis of Halobacterium halobium gas vesicles. J Bacteriol. 1973 Jun;114(3):1058–1067. doi: 10.1128/jb.114.3.1058-1067.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen H., Omang S., Steensland H. On the gas vacuoles of the halobacteria. Arch Mikrobiol. 1967;59(1):197–203. doi: 10.1007/BF00406332. [DOI] [PubMed] [Google Scholar]
- Staley J. T. Prosthecomicrobium and Ancalomicrobium: new prosthecate freshwater bacteria. J Bacteriol. 1968 May;95(5):1921–1942. doi: 10.1128/jb.95.5.1921-1942.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ert M., Staley J. T. Gas-vacuolated strains of Microcyclus aquaticus. J Bacteriol. 1971 Oct;108(1):236–240. doi: 10.1128/jb.108.1.236-240.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waaland J. R., Branton D. Gas vacuole development in a blue-green alga. Science. 1969 Mar 21;163(3873):1339–1341. doi: 10.1126/science.163.3873.1339. [DOI] [PubMed] [Google Scholar]
- Waaland J. R., Waaland S. D., Branton D. Gas vacuoles. Light shielding in blue-green algae. J Cell Biol. 1971 Jan;48(1):212–215. doi: 10.1083/jcb.48.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsby A. E. Structure and function of gas vacuoles. Bacteriol Rev. 1972 Mar;36(1):1–32. doi: 10.1128/br.36.1.1-32.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]