Abstract
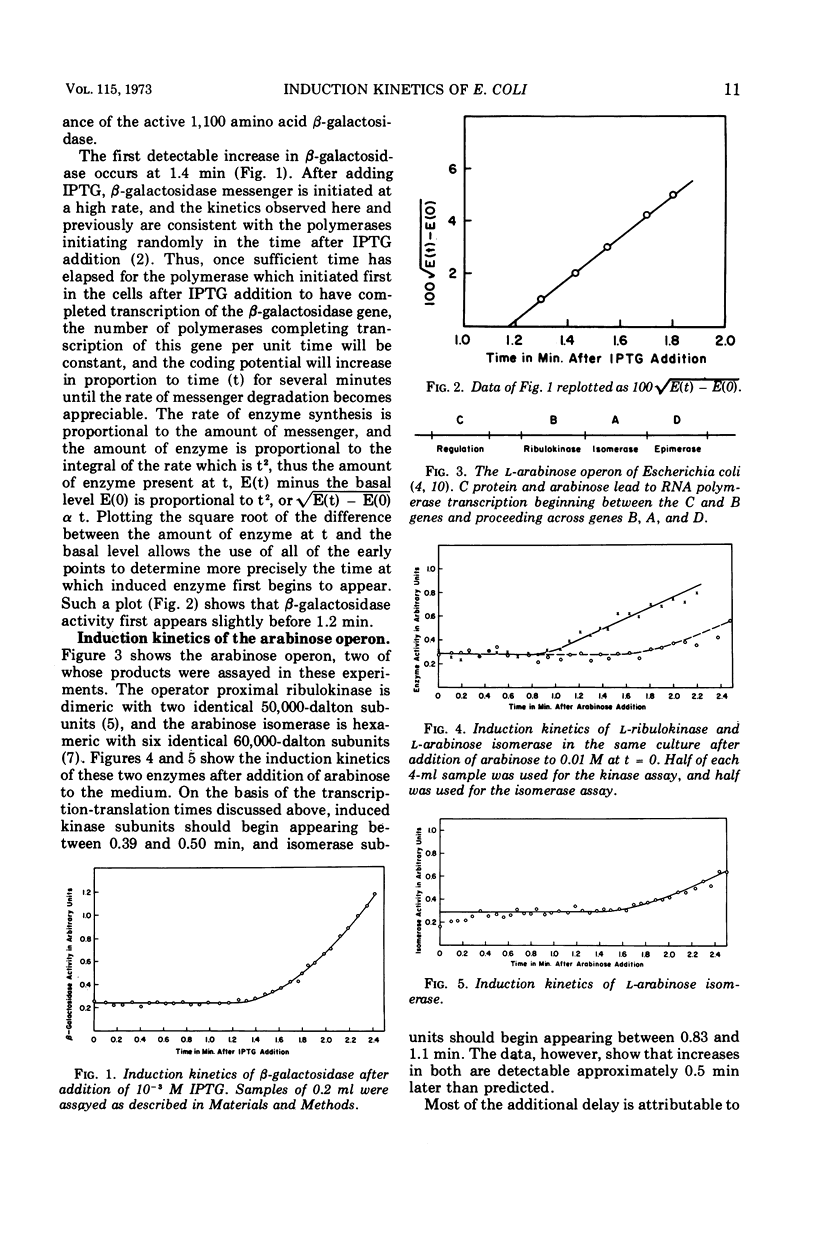
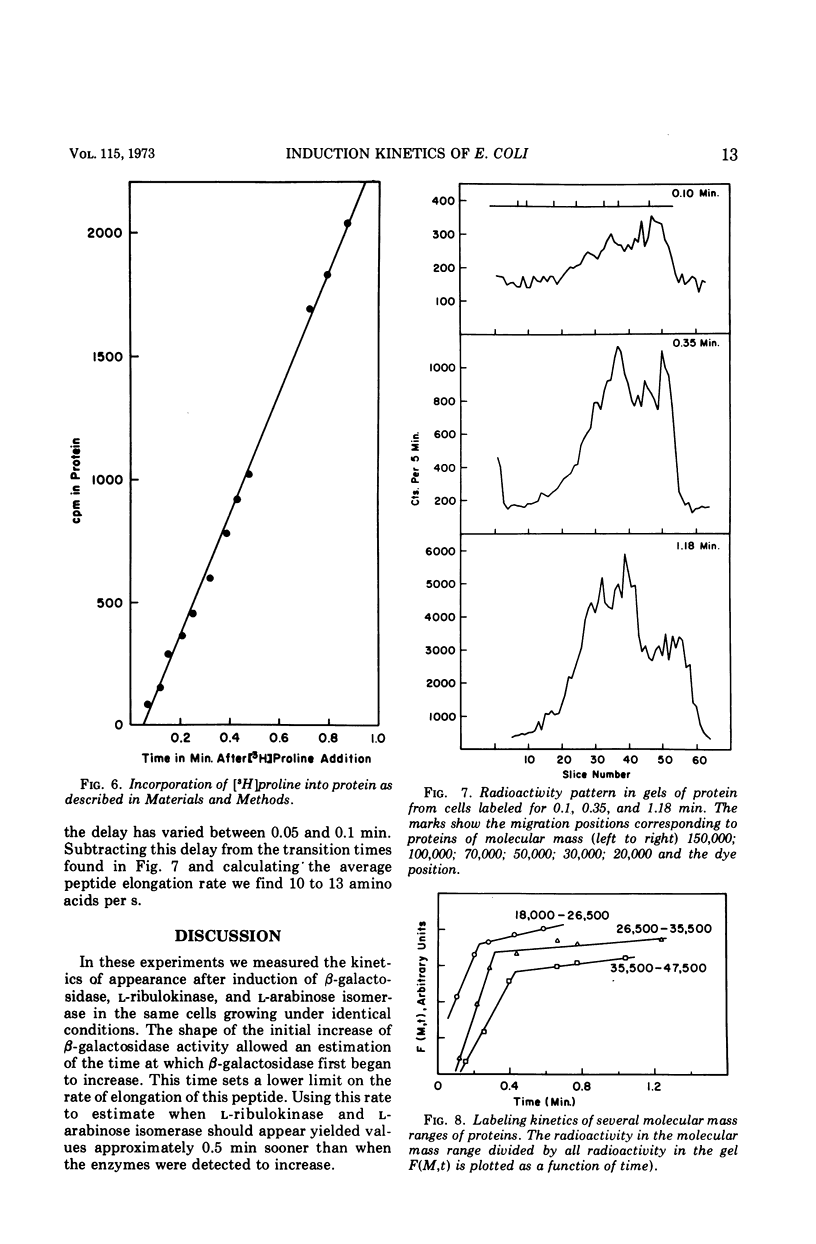
After addition of l-arabinose to growing Escherichia coli, the l-ribulokinase (EC 2.7.1.16) and l-arabinose isomerase (EC 5.3.1.4) first appear at about 0.7 and 1.4 min, respectively. These times are consistent with the distances of the genes from the ribonucleic acid polymerase initiation site in the operon. The kinetics of appearance of these enzymes as well as those of β-galactosidase (EC 3.2.1.23) in the same strain are consistent with a peptide elongation rate of no less than 14 amino acids per second. A measurement of the average peptide elongation rate made by measuring the kinetics of radioactive amino acid appearance in completed polypeptides yielded a rate of about 12 amino acids per s. Convenient assays of the arabinose isomerase and ribulokinase are also given.
Full text
PDF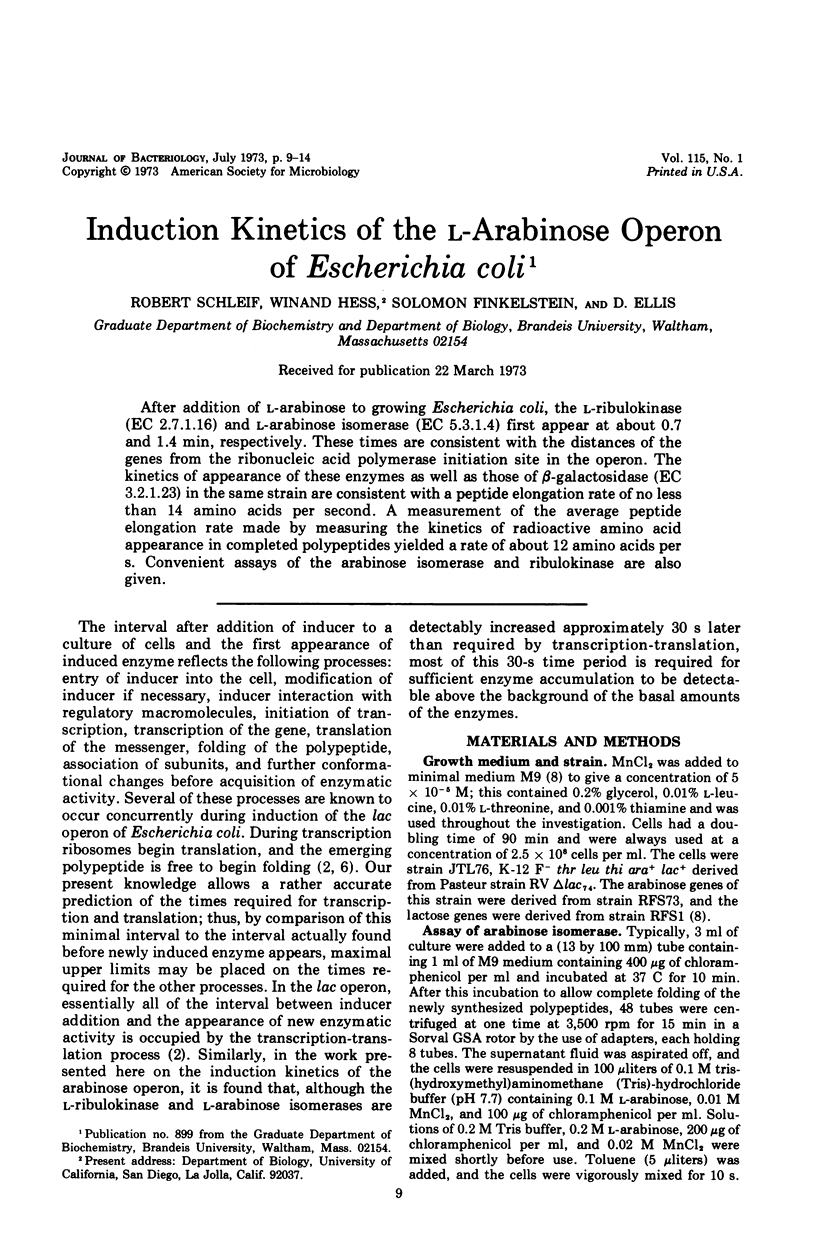



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bremer H., Yuan D. RNA chain growth-rate in Escherichia coli. J Mol Biol. 1968 Dec 14;38(2):163–180. doi: 10.1016/0022-2836(68)90404-x. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Norris T. E., Koch A. L. Chain elongation rate of messenger and polypeptides in slowly growing Escherichia coli. J Mol Biol. 1971 Aug 28;60(1):1–19. doi: 10.1016/0022-2836(71)90442-6. [DOI] [PubMed] [Google Scholar]
- GROSS J., ENGLESBERG E. Determination of the order of mutational sites governing L-arabinose utilization in Escherichia coli B/r bv transduction with phage Plbt. Virology. 1959 Nov;9:314–331. doi: 10.1016/0042-6822(59)90125-4. [DOI] [PubMed] [Google Scholar]
- Gausing K. Efficiency of protein and messenger RNA synthesis in bacteriophage T4-infected cells of Escherichia coli. J Mol Biol. 1972 Nov 28;71(3):529–545. doi: 10.1016/s0022-2836(72)80021-4. [DOI] [PubMed] [Google Scholar]
- Lee N., Bendet I. Crystalline L-ribulokinase from Escherichia coli. J Biol Chem. 1967 May 10;242(9):2043–2050. [PubMed] [Google Scholar]
- Leive L., Kollin V. Synthesis, utilization and degradation of lactose operon mRNA in Escherichia coli. J Mol Biol. 1967 Mar 14;24(2):247–259. doi: 10.1016/0022-2836(67)90330-0. [DOI] [PubMed] [Google Scholar]
- Patrick J. W., Lee N. Subunit structure of L-arabinose isomerase from Escherichia coli. J Biol Chem. 1969 Aug 25;244(16):4277–4283. [PubMed] [Google Scholar]
- Schleif R. An L-arabinose binding protein and arabinose permeation in Escherichia coli. J Mol Biol. 1969 Nov 28;46(1):185–196. doi: 10.1016/0022-2836(69)90065-5. [DOI] [PubMed] [Google Scholar]
- Schleif R. Fine-structure deletion map of the Escherichia coli L-arabinose operon. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3479–3484. doi: 10.1073/pnas.69.11.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleif R., Greenblatt J., Davis R. W. Dual control of arabinose genes on transducing phage lambda-dara. J Mol Biol. 1971 Jul 14;59(1):127–150. doi: 10.1016/0022-2836(71)90417-7. [DOI] [PubMed] [Google Scholar]



