Abstract
The σ-N (σN) subunit of the bacterial RNA polymerase is a sequence specific DNA-binding protein. The RNA polymerase holoenzyme formed with σN binds to promoters in an inactive form and only initiates transcription when activated by enhancer-binding positive control proteins. We now provide evidence to show that the DNA-binding activity of σN involves two distinct domains: a C-terminal DNA-binding domain that directly contacts DNA and an adjacent domain that enhances DNA-binding activity. The sequences required for the enhancement of DNA binding can be separated from the sequences required for core RNA polymerase binding. These results provide strong evidence for communication between domains within a transcription factor, likely to be important for the function of σN in enhancer-dependent transcription.
The first step in gene expression, transcription initiation, is tightly regulated at the level of RNA polymerase activity (1). A specialized form of RNA polymerase found in bacteria functions in enhancer-dependent transcription and employs activator proteins that possess a nucleoside triphosphatase activity required for their positive control function (2–5). The sigma-N (σN) subunit of the RNA polymerase is necessary and sufficient to modify core RNA polymerase so as to form the specialized enhancer-responsive holoenzyme (6–10). Prokaryotic transcription that is independent of σN does not require β–γ bond nucleotide hydrolysis but both prokaryotic and eukaryotic enhancer-dependent transcription does. Promoter-specific DNA-binding activity of σN is central to formation of the anisometric RNA polymerase–promoter complex that is the target of activator proteins (11, 12).
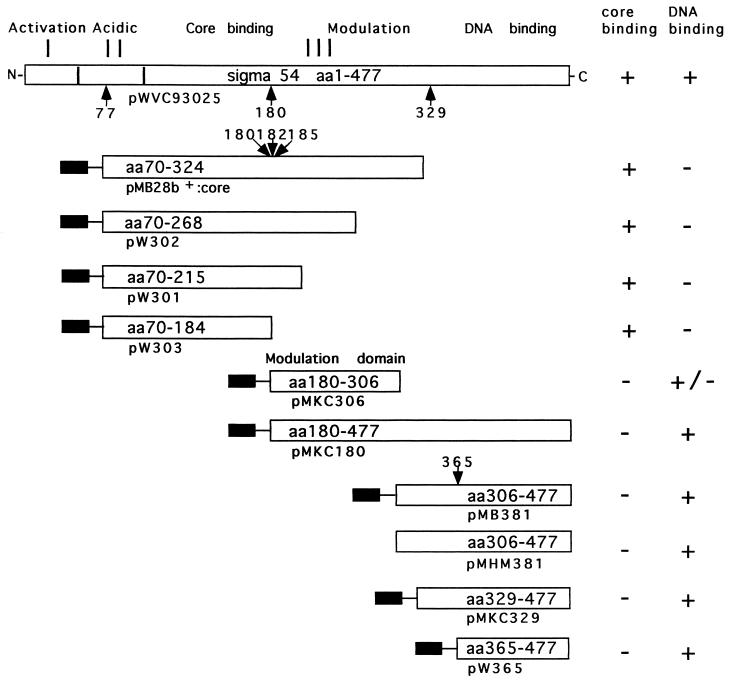
A domain model for σN has been elaborated (13–15; see Fig. 1) and certain conserved residues that are important for retention of activity have been identified (16–19). However, little is known about intramolecular interactions within σN or how such interactions might contribute to activity. The C-terminal 329–477 residues of σN include the DNA-binding domain, which contacts consensus DNA sequences comprising the recognition sequence of the σN holoenzyme (14, 15, 17, 20, 21). The DNA contacted by σN and its holoenzyme includes sequences that are melted upon formation of an open promoter complex (22), suggesting a role for σN in DNA melting (23). We now report evidence strongly suggesting that the DNA-binding activity of σN is modulated by an intramolecular interaction between domains of σN. This conclusion is based upon measurements of the in vitro DNA-binding activity of the DNA-binding domain of σN and enhancement of this activity by adjacent amino acid sequences that appear to be a separate unit of folding and therefore a discrete domain (see Fig. 1). The adjacent sequences functioned to increase DNA binding when present as either a separate polypeptide or when linked to the DNA-binding domain. The activity responsible for enhancing and potentially modulating DNA-binding activity is present within a part of σN distinct from that part which binds core RNA polymerase (see Fig. 1). Results are discussed with respect to an architecture of σN needed for enhancer-dependent transcription.
Figure 1.
The primary organization of σN and its functional domains (8, 14, 15). Amino acids 1–477 of the K. pneumoniae protein can be divided into regions I–III (13). Region I is glutamine-rich and involved in activation of the holoenzyme (28–30), region II is acidic and is variable among members of the σN protein family (8, 23) and region III includes the core binding determinant, the DNA-binding domain, and the domain described in this paper which enhances and modulates DNA-binding activity (8, 14, 15, 18). Arrows mark proteolytically sensitive sites (21) relevant to this work, and the amino acid residue C terminal to the cleavage site is indicated. The site at 365 was established by chymotryptic challenge of purified peptides comprising amino acids 329–477 and 306–477 and searching for common peptides and the site at 185 by tryptic challenge of the 70–324 peptide (X.-Y.W. and M.B., unpublished data); the other sites have been previously reported (15, 21). Protein fragments employed in this work are shown and their activities are indicated. Plasmids directing peptide synthesis are indicated below each protein fragment, and the solid bar the N-terminal extension-MGSSH6SSGLVPRGSHM arising from the vector. In the case of the 365–477 and 306–477 peptides the Met at 365 and at 306 is contributed by this extension. The activity that enhances DNA-binding resides in peptide 180–306 approximates a 180–303 chymotryptic peptide of σN (15, 21).
MATERIALS AND METHODS
Plasmid Constructions.
Constructs are summarized in Fig. 1. To overproduce the 30-kDa core binding peptide of σN, identified by proteolysis as an active domain (15), a SalI–PstI fragment from the Klebsiella pneumoniae rpoN gene (24) and encoding amino acids 70–324 was cloned into a modified PT7-7 vector (PT7-7:4803/4804) restricted with SalI and PstI to generate PMB7-7:760. The PT7-7:4803/4804 vector was generated by ligating between the PstI and HindIII sites of PT7-7 the linker formed by annealing oligonucleotides 5′-GTGATGATATCA and 5′-AGCTTGATATCATCACTGCA so as to introduce tandem UGA stop codons after the PstI site. From PMB7-7:760 the unique NdeI–HindIII fragment encoding amino acids 70–324 of σN was isolated and cloned into PET28b+ (Novagen) restricted with NdeI and HindIII forming PMB28b+:core which directs the synthesis of an amino-terminal 6-His-tagged σN peptide. Deletion derivatives of the rpoN sequence in PMB28b+:core were obtained by amplifying parts of the sequence using the T7 primer to read through the NdeI site and another primer designed to (i) truncate at amino acids 268, 215, and 184 by inclusion of an UGA termination codon and (ii) introduce a HindIII site downstream of the termination codon. Following thermal cycle amplification, the product of the reaction was restricted with NdeI and HindIII, gel purified, and cloned into PET28b+ also restricted with NdeI and HindIII. Plasmids PW301, -302, and -303 constructed as above directed the synthesis of N-terminal 6-His-tagged polypeptides specifying residues 70–215, 70–268, and 70–184 of σN.
The gene fragment encoding the DNA-binding domain of σN was cloned using thermal cycle amplification to introduce an NdeI site at the codon corresponding to amino acid 329 or 365 of σN. Plasmid PMM70 (a PTZ19 clone of the EcoRI–HindIII rpoN-containing fragment of PMM17; ref. 24) was used as template DNA and the reverse sequencing primer was used to prime DNA synthesis in the opposite direction to the NdeI site primers. The amplified DNA fragments were restricted with NdeI and HindIII and cloned into PET28b+ also restricted with NdeI and HindIII. Plasmids PMKC329 and PW365 direct the synthesis of amino acids 329–477 and 365–477 of σN respectively with 6-His N-terminal tags. Similarly PMKC180 directing the synthesis of amino acids 180–477 and PMKC306 directing synthesis of amino acids 180–306 were prepared. To His-tag the 306–477 DNA-binding peptide (15), synthetic NdeI–NcoI linkers flanking a poly-His encoding sequence (5′-(CAT)6ACC) were introduced between the NdeI and NcoI-specified ATG codons of PT7-7 and PMHM381 (15), respectively. The resulting plasmid PMB381.1 carries the NcoI–BamHI fragment of PMHM381 (encoding amino acids 306–477) in pT7-7.
Protein Overproduction and Purification.
Purified proteins and peptides are summarized in Fig. 1. Plasmids directing overproduction of K. pneumoniae σN peptides were transformed into Escherichia coli BL21/DE3 and cells were grown at 30°C (for overproduction of amino acids 70–324 and truncated derivatives) or at 37°C for overproduction of the σN DNA-binding domain (amino acids 306–477, 329–477, 365–477) and σN amino acids 180–477. Bacteria were grown in 1-liter batches from 10–20% inoculation and induced after 3–4 h growth by the addition of 1 mM isopropyl β-d-thiogalactoside. Cells were harvested after 3–4 h induction, resuspended in 50 mM Tris⋅HCl (pH 8.0), 50 mM NaCl, and 5% glycerol, and broken by three cycles of freeze thaw in the presence of 1% (wt/vol) sodium deoxycholate, 1 mM DTT, 0.1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride (PMSF), and 20 μg/ml lysozyme. Following centrifugation at 20,000 × g for 30 min the DNA-binding domain peptides and the 180–306 and 180–477 peptides were found in the insoluble cell fraction where as the 70–324 peptide and its derivatives starting at amino acid 70 were found predominantly in the soluble fraction. The soluble σN peptides were purified by Ni affinity chromatography at pH 7.0 after dialysis into 25 mM sodium phosphate, 0.5 M NaCl, and 5% glycerol and eluted with an imidazole gradient. Further purification was achieved by chromatography on a 1 ml Resource Q (Pharmacia) column using a NaCl gradient in 20 mM imidazole (pH 7.0), 1 mM DTT, 0.1 mM EDTA, and 5% glycerol. Pooled peak fractions were dialyzed into 50 mM Tris⋅HCl (pH 8.0), 50 mM NaCl, 0.1 mM EDTA, 1 mM DTT, and 50% glycerol for storage at −70°C. Following extraction and washing of the insoluble cell fraction with 1 M NaCl and 1% Triton X-100 the DNA-binding peptides were solubilized in 50 mM Tris⋅HCl (pH 8.0), 50 mM NaCl, 0.1 mM EDTA, 1 mM DTT, 5% glycerol, 1% sarkosyl, and 1 mM PMSF (15). The sarkosyl was removed by dialysis against 50 mM Tris⋅HCl (pH 8.0), 250 mM NaCl, 0.1 mM EDTA, 1 mM DTT, 5% glycerol, and 1 mM PMSF for 15 h at 4°C and then for a further 4 h with the NaCl concentration reduced to 100 mM. Solublized material was exchanged into 25 mM phosphate buffer (pH 7.0) and 0.5 M NaCl, subjected to Ni affinity chromatography and elution with an imidazole gradient. Peak fractions were pooled and dialyzed in 50 mM Tris⋅HCl (pH 8.0), 0.5 M NaCl, 0.1 mM EDTA, 1 mM DTT, and 50% glycerol for storage. DNA-binding peptides were also diluted in this buffer prior to assay. The 180–306 peptide was refolded from urea (15) and following Ni affinity purification was stored in 50 mM Tris⋅HCl (pH 8.0), 100 mM NaCl, 0.1 mM EDTA, 1 mM DTT, and 50% glycerol.
Core RNA Polymerase Binding Assays.
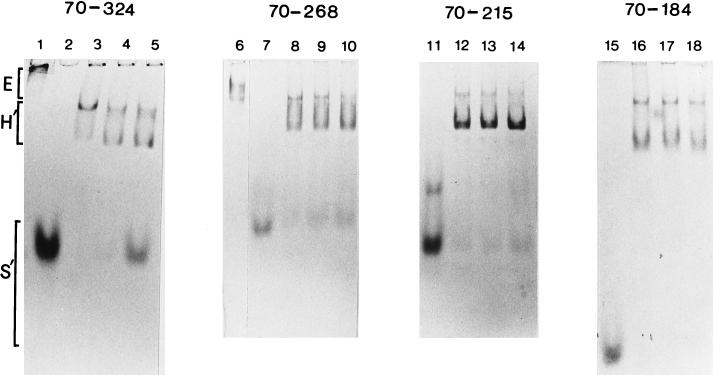
These were performed as described (15, 25) except that 4.5% native polyacrylamide gels (37.5:1, acrylamide/bis-acrylamide) prepared as 7 cm × 10 cm, 1 mm-thick gels were employed to separate core RNA polymerase, holoenzyme, and σN peptides. Proteins were visualized by Coomasie staining. Assays (8 μl) contained 300 nM core RNA polymerase and different amounts of σN peptides (see Fig. 3 legend). Gels were run at 50 V cm−1 for 2 h at room temperature in 25 mM Tris/200 mM glycine buffer, pH 8.6.
Figure 3.
Binding of the 70–184, 70–215, 70–268, and 70–324 peptides to core RNA polymerase. A constant amount of core (E) was combined with increasing amounts of σ peptides (S′). Formation of a holoenzyme (H′) was detected as the presence of a faster migrating species when compared with E alone (lanes 1 and 6). All σ peptides formed a holoenzyme complex at a ratio of 1:1 with E (lanes 3, 8, 12, and 16). Other ratios E/S′) were 1:4 (lanes 4, 9, 13, and 17) and 1:10 (lanes 5, 10, 14, and 18). Free σ peptides (lanes 2, 7, 11, and 15) are also shown. In a separate experiment (data not shown), on native gels the order of mobility for the free σ peptides was 70–324 = 70–184 < 70–268 < 70–215.
DNA Interaction Assays.
These were conducted using the Rhizobium meliloti nifH promoter as the target DNA sequence (26). Briefly, single-stranded DNA from an M13 clone was extended with Klenow polymerase using a 5′ 32P-labeled universal primer and then restricted to generate defined length end-labeled double stranded DNA for footprinting. Exonuclease III and DNase I footprinting was then conducted to detect binding of σN peptides to promoter DNA (20, 27). Binding reactions (25–50 μl, 30°C) were conducted in 25 mM Tris-acetate (pH 8.0), 8 mM Mg acetate, 10 mM KCl, 1 mM DTT, and 3.5% (wt/vol) PEG 6000 and contained 1.2–2.4 nM template DNA (27) and the amount of σN peptide indicated in the figure legends. Protein solutions contributed 2–4% of the final assay volume. Proteins and DNA were incubated for 15–20 min before the addition of 175 units of exonuclease III (Pharmacia) for 3 min or 0.0035 units of DNase I (Amersham) for 45 sec. Reactions were terminated by rapid phenol extraction. Nucleic acids were recovered by ethanol precipitation, electrophoresed through 6% denaturing polyacrylamide gels that were then dried and autoradiographed. Markers were generated by chemical cleavage of the DNA with piperidine following partial methylation with dimethyl sulfate (28).
RESULTS
DNA-Binding Activities of σN Peptides.
Previous work had established that amino acids 329–477 of σN bound specifically to promoter DNA sequences recognized by σN and its holoenzyme (15, 21). The 329–477 DNA-binding peptide was shown to interact with amino acids 107–303 of σN by the criteria of copurification and chemical crosslinking of the two peptides to each other (15). To examine the potential influence of intramolecular interactions on σN function a series of DNA binding and adjacent peptides were purified (Fig. 1). The choice of the N- and C-terminal boundaries of these peptides was guided by the identification of proteolytically sensitive sites in σN as potential structural domain boundary markers (15, 21). Briefly, in this work sites at amino acids 70, 180, 185, 306, 329, and 366 were chosen and convenient restriction sites or primer generated end points were used to construct deletions of the rpoN gene (Fig. 1 and Materials and Methods).
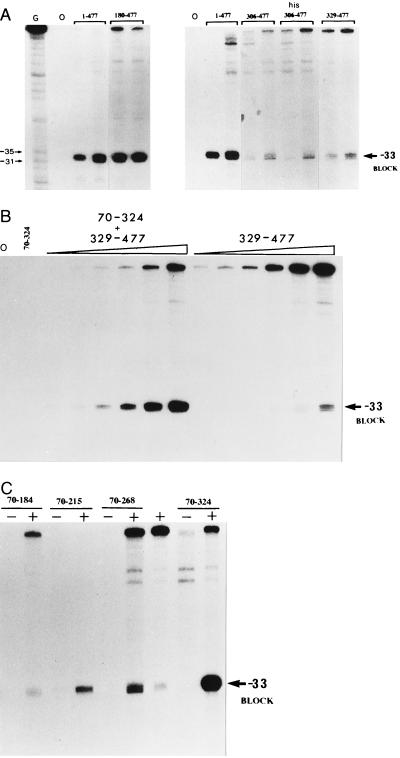
The double-stranded DNA-binding activities of peptides comprised of amino acids 180–477, 306–477 (6-His-tagged and nontagged), and 329–477 of σN were examined by exonuclease III footprinting (Fig. 2A) and compared with σN. In this assay the binding is detected by a block to exonuclease III digestion at −33 on the template strand (15, 20). As judged by the intensity of the footprint the DNA-binding activity of the 180–477 peptide preparation was significantly higher than that of the DNA-binding domain peptides 306–477 and 329–477, and approximated the activity of the full-length σN protein (data not shown). The 70–324 peptide at up to 5 μM lacked detectable DNA-binding activity consistent with its lack of residues 329–477 which are involved in DNA contact (but see below). The greater DNA-binding activity of the 180–477 peptide compared with the 329–477 peptide indicated that residues between 180 and 328 could be enhancing the DNA-binding activity of the 329–477 DNA-binding domain.
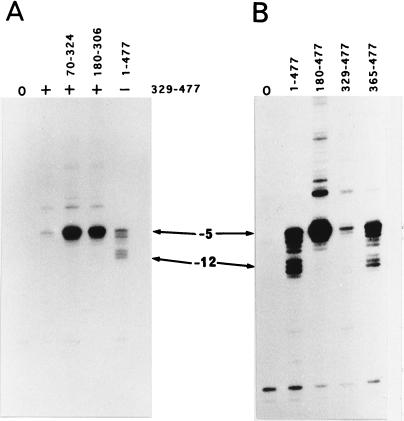
Figure 2.
(A) Exonuclease III footprints of the 1–477 σN protein and 180–477, 306–477 (non-His and His-tagged), and 329–477 peptides each at 250 mM and 1 μM. On the basis of the intensity of the block at −33 the σN and 180–477 peptides displayed stronger binding than the 306–477 and 329–477 peptides. (B) Increase of the −33 exonuclease III footprint signal in a combined DNA-binding assay. The presence of 1 μM 70–324 peptide combined with the 329–477 peptide (25 nM, 50 nM, 100 nM, 250 nM, 500 nM, and 1 μM) produced a significantly stronger footprint signal compared with single protein footprints (1 μM). The 70–324 peptide was added to the assay mix prior to the 329–477 peptide. (C) Combined exonuclease III footprint with the 329–477 peptide (+) at 250 nM and a series of peptides (70–184, 70–215, 70–268, 70–324) at 1 μM. Peptides were premixed (20–25 μM) before addition of the DNA.
It was consistently observed that the 306–477 and 329–477 peptides protected the end of the DNA footprinting template from exonuclease digestion, indicating some single-strand DNA-binding activity (data not shown). End protection was not as evident with the 365–477 peptide (see Fig. 4B), suggesting the single-strand binding activity is closely associated with amino acids 329–364. The full-length σN does not show strong end protection suggesting that the single-strand binding activity may be reduced by the presence of other σN sequences.
Figure 4.
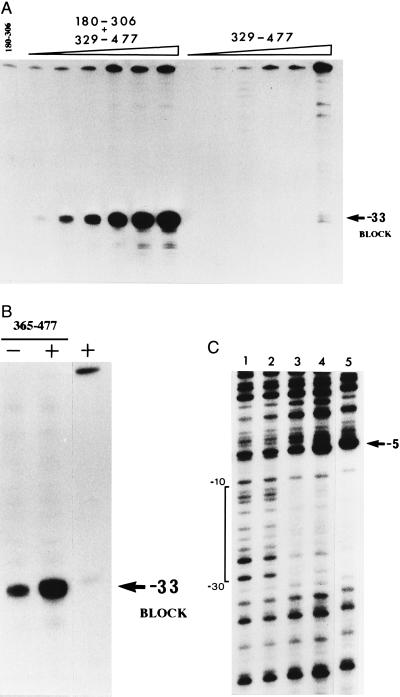
(A) Increase of the −33 exonuclease III footprint signal in a combined DNA-binding assay. The presence of 1 μM 180–306 peptide with the 329–477 peptide (25 nM, 50 nM, 100 nM, 250 nM, 500 nM, and 1 μM) produced a significantly stronger signal compared with single peptide footprints. The 180–306 peptide was added to the assay mix before the 329–477 peptide. (B) Increase of the −33 exonuclease III footprint signal in a combined DNA-binding assay in the presence of 1 μM 180–306 peptide (+) and 1 μM 365–477 peptide. The peptides were premixed (25 μM) before addition of the DNA. The increased signal of the 365–477 peptide compared with the 329–477 peptide (Fig. 2) is attributable in part to reduced protection of the DNA fragment end. (C) Increase of the DNase I footprint signal in a combined assay containing the 329–477 peptide and 180–306 (lane 4) or 70–324 (lane 3) peptides each at 2 μM. The extent of protection was the same as for σN (lane 5). Peptides were premixed as in Fig. 2C before assay (lane 1, no protein). The 329–477 peptide was footprinted alone (4 μM) in lane 2 and protected the −24 part of the promoter weakly compared with the combined assays (lanes 3 and 4). Protected DNA sequences are bracketed.
Enhancement of the DNA-Binding Domain’s Activity.
To examine the influence of sequences N terminal to position 329 upon DNA binding, the 70–324 and 329–477 peptides were both included in an exonuclease III footprint. Results of this experiment demonstrated that the 70–324 peptide greatly increased the footprint signal seen with the 329–477 peptide alone (Fig. 2B, and Table 1). Increased footprints were seen when template (Fig. 2B) and nontemplate strands were assayed (see Fig. 5A). The extent of the footprint as judged by blocks at −33 and −5 remained the same as for the DNA-binding domain, indicating that enhancement of DNA binding did not involve DNA contacts outside of those made by the 329–477 DNA-binding domain peptide.
Table 1.
Combinations of σ peptides that gave increased DNA footprints compared to single peptide footprints
| Sigma peptides
| ||
|---|---|---|
| DNA-binding | Enhancing | |
| 329–477 | + | 70–324 |
| 329–477 | + | 180–306 |
| 323–477 | + | 70–324 |
| 323–477 | + | 180–306 |
| 365–477 | + | 180–306 |
Figure 5.
Exonuclease III footprints of the top strand showing blocks around −5 and −12. Combined DNA-binding assays (A) were conducted in the presence of the 329–477 peptide (+) with either 70–324 or 180–306 peptides and compared with 329–477 peptide alone and to σN (1–477). Top strand contacts made by 180–477, 329–477, 365–477, and σN are shown in B. All proteins were used at 1 μM concentration.
Core RNA Polymerase Binding Can Be Separated from the Enhancement of DNA Binding.
Previous work had indicated that the 77–311 peptide liberated from σN by proteolysis and which bound core RNA polymerase is comprised of two structural domains (15). Sites around amino acid 180 were sensitive to proteolysis (Fig. 1) and a peptide comprising of amino acids 180–303 appeared as a stable product of proteolysis (15). Similar results were obtained when the overproduced 70–324 peptide was proteolysed (X.-Y.W. and M.B., unpublished data). In principle a fragment derived from the 70–324 sequence could stimulate DNA binding. By constructing a series of truncated derivatives of the 70–324 peptide we established that amino acids 70–184 failed to enhance DNA binding in the combined footprint assay (Fig. 2C) but retained core RNA polymerase binding (Fig. 3). A clear increase of the exonuclease III footprint signal was seen with the 70–324 peptide, weakly with the 70–268 and 70–215 peptides, but not with the 70–184 peptide (Fig. 3). These results establish that the core RNA polymerase binding and DNA-binding enhancement activities can be separated and strongly suggest that the latter activity resides between amino acids 184–324. Using a number of proteins unrelated to σN in combination with the 329–477 peptide we were unable to demonstrate an increased footprint, arguing for a specific effect of residues 184–324 upon DNA binding (data not shown).
A Discrete Domain of σN Enhances DNA Binding.
Deletion analysis of the 70–324 core-binding and footprint enhancing peptide established that the core binding activity could be separated from the activity enhancing DNA binding. Amino acids 180–306 correspond to a previously reported subdomain of a core-binding σN peptide (15) and include those amino acids that when deleted from the 70–324 peptide result in a loss of the enhancement of DNA binding (see Fig. 3). To determine whether the enhancement activity resided within a discrete domain of σN, the 180–306 peptide was overexpressed and purified for use in a combined DNA footprinting experiment. As reported (15) for the 180–303 peptide liberated by proteolysis of σN, the overproduced 180–306 peptide bound to heparin matrix indicating that the overproduced and native peptides have some similar character. The 180–306 peptide failed to form a detectable complex with core RNA polymerase (data not shown) consistent with the view that important core-binding determinants lie between amino acids 77 and 180 (15). Similarly the amino acids 180–477 peptide failed to bind to core RNA polymerase (data not shown).
In a combination footprint experiment the 329–477 and 180–306 peptides together produced a greatly increased footprint (Fig. 4A, Table 1). The enhancement of binding was at least equal to the enhancement seen with the 70–324 peptide (Fig. 2B) and was also clearly evident when the natural 329–477 DNA-binding peptide liberated by proteolysis of σN (15) replaced the refolded 329–477 peptide (data not shown). Enhancement of binding afforded by the 180–306 peptide was also evident with the 365–477 peptide (Fig. 4B and Table 1), indicating that the increased footprint does not absolutely require amino acids 329–364. However, the enhancement of DNA binding was not as strong as with the 329–477 peptide, suggesting that the 329–364 sequence does contribute to the enhanced footprint. By the criterion of being an independently folded unit of protein structure the results establish that amino acids 180–306 comprised a domain of σN with a discrete activity closely associated with enhancing DNA binding but apparently not with core RNA polymerase binding.
Interestingly, the amino acids 180–306 domain itself produced a weak exonuclease III footprint when assayed (Fig. 4B) indicating that its contribution to the increased footprint could involve direct DNA contact as well as the protein contact with the 329–477 domain as previously detected by crosslinking of the two domains (15). Judged by the intensity of the exonuclease footprint the 180–306 peptide bound about one-fiftieth as well as the 329–477 peptide. DNA binding by the 180–306 peptide was observed with three independent preparations and was sequence specific by the criteria of the location of the exonuclease block at −33. Repeated experiments failed to detect DNA binding by the 70–324 peptide indicating that the DNA-binding activity associated with amino acids 180–306 could be masked within the 70–324 peptide.
Order of addition experiments were conducted to examine whether evidence, in addition to previous crosslinking results (15), in favor of complex formation between the 329–477 peptide and the 180–306 peptide could be obtained. When the peptides were pre-incubated together rather than being added separately to the DNA target the footprint was stronger (data not shown), a result consistent with DNA binding by a preformed complex between the 329–477 and 180–306 peptides. In the light of the above results we cannot discount the possibility that previous footprints with the proteolytically liberated 329–477 peptide (15) may have some increased signal attributable to a low level of a contaminating peptide related to the 180–306 sequence.
Enhanced Binding Involves Similar Contact to Promoter DNA.
DNase I footprinting (Fig. 4C) demonstrated that the extent of contact made in the increased footprint was very similar to that made by σN and further established that the footprint made by the 329–477 peptide was very much weaker than that obtained in the combined footprint (Fig. 4C). The increased footprint differed qualitatively from that of σN in that the hyperactivity toward the base at −5 was weaker. Because core RNA polymerase assists binding of σN we included core in a DNase I combined footprinting assay with the 70–324 core binding peptide and the 329–477 DNA-binding peptide. No core-dependent increase in the intensity of the footprint was observed (data not shown). However the −5 hyperreactivity was weakened relative to the control without core [resulting in a footprint qualitatively very similar to that seen with an N-terminal delete σN (107–477) holoenzyme (15)], indicating that core binding influenced the σ peptides–DNA interaction somewhat.
The top strand contact by σ peptides was examined using exonuclease III (Fig. 5). The increased footprint was evident as a stronger block at −5 when the 329–477 peptide was assayed in combination with the 70–324 or 180–306 peptides (Fig. 5A). The 180–477 peptide also gave a strong −5 block (Fig. 5B). The increased footprints did not extend beyond the boundaries of contacts made by the the 329–477 and 365–477 peptides at −33 and −5. However the increased 329–477 and 180–477 footprints all differed from the σN and 365–477 footprints in that the −12 block was not as evident. These differences can be ascribed in part to the amino region I of σN that influences DNA contact made by the σ around the −12 promoter element (14, 15, 29, 30) and also to residues 329–364 which distinguish the 329–477 and 365–477 peptides. Residues 329–364 include a site of DNA crosslinking (between 332 and 345) and additionally several acidic residues and so might contribute both positively and negatively to DNA binding.
DISCUSSION
Recognition of promoter DNA by σN is a key activity necessary for locating the RNA polymerase holoenzyme at the transcription start site (1, 31, 32). DNA contact by σN is also likely to be involved in DNA melting (22, 23), a step in σN-dependent transcription accelerated by the activator protein (10). Our results support a model for the σN DNA-binding function (15) in which a discrete domain (residues 180–306) assists the DNA binding of a separate domain responsible for directly contacting the consensus elements of the promoter (see Fig. 1 and Table 1). Thus DNA binding by σN appears to be specified by two structural domains. Two additional independent lines of evidence also argue that the 180–306 domain functions to assist DNA binding, likely through an intramolecular interaction. First, an internal deletion analysis of σN demonstrated that disruption of the 180–306 domain (through deletion of amino acids 246–291) resulted in a loss of DNA-binding activity, consistent with our results establishing that the 180–306 domain enhances DNA binding, illustrating that the domain functions to assist DNA binding within the full-length protein (14). Second, σN undergoes a two-step cooperative melting transition that is dependent upon an intact DNA-binding domain and upon residues 180–306, but which is independent of N-terminal residues (S. Missailidis and M.B., unpublished data). It would appear that amino acids 304–328 which link the DNA binding and enhancing domains comprise a surface exposed element in σN since proteoloytically sensitive sites map here (ref. 21; P. Casaz and M.B., unpublished data). The minimum function of the element is to link two domains. Other roles for the linking element are possible, and include activator contact (11) and signal transduction through σN.
Previous protein crosslinking experiments (15) suggest that one mechanism by which the 180–306 domain could enhance DNA binding would involve a protein-protein interaction with the 329–477 domain. A DNA contact by the 180–306 domain may also be involved in establishing the enhanced DNA binding since a weak exonuclease III footprint was consistently observed with the 180–306 domain. Because the interaction between σN and DNA changes throughout transcription (33) and is also conditioned by the binding of core RNA polymerase to σN (20, 25, 30), the 180–306 domain is likely to be involved in determining how σN interacts with DNA during the σN transcription cycle. Intramolecular communication within transcription factors is a relatively poorly described but potentially very important feature of their function and is likely to be involved in establishing alternate conformational states and in signal transduction through σN. Evidence from in vitro footprinting work suggests that σN binds DNA in an altered conformational state following the initiation of transcription (33). Furthermore, mutant σN proteins that direct activator-independent transcription show an altered contact to promoter DNA (34–36). Activation of the σN holoenzyme presumably involves changes in interactions between protein domains. The 180–306 sequence is a candidate domain involved in the signal transduction pathway that exists between the binding of activator, formation of an open promoter complex, and the initiation of transcription. Ligands modifying the activity of σN might also work through the activity of the 180–306 domain (8). Taken together with previous domain analyses (14, 15), it appears that σN comprises four functional domains: activation, core binding, enhancement/modulation of DNA binding, and DNA contact from N to C terminus, respectively (see Fig. 1 and ref. 15). The domains are likely to interact via intramolecular contacts to establish the full specialized function of σN in enhancer-dependent transcription (10, 37, 38). Specifically, this is the ability to form a stable but transcriptionally inactive closed promoter complex that responds to remotely bound activators. The activation and modulation domains, apparently absent from the σ70 class proteins that bind promoters in a transcriptionally active form (1, 39), may be key in establishing enhancer-dependent transcription through a specialized σ architecture critically involving the activation and modulation domains.
Acknowledgments
We wish to thank Paul Casaz and Barrie Davidson for useful comments on the manuscript and helpful discussions throughout the work, Arno Karnholz and Loiuse Kim for help with peptide purification, and M. Merrick for providing PMM70. The work was supported by the Wellcome Trust. M.K.C. was supported by a Biotechnology Biological Sciences Research Council studentship and X.-Y.W. by funding from the European Community.
References
- 1.Gross C A, Lonetto M, Losick R. In: Transcriptional Regulation. Yamamoto K, McKnight S, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1992. pp. 129–176. [Google Scholar]
- 2.Weiss D S, Batut J, Klose K E, Keener J, Kustu S. Cell. 1991;67:155–167. doi: 10.1016/0092-8674(91)90579-n. [DOI] [PubMed] [Google Scholar]
- 3.Austin S, Dixon R A. EMBO J. 1992;11:2219–2228. doi: 10.1002/j.1460-2075.1992.tb05281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee J H, Scholl D, Nixon B T, Hoover T R. J Biol Chem. 1994;269:20401–20409. [PubMed] [Google Scholar]
- 5.Austin S, Buck M, Cannon W, Eydmann T, Dixon R. J Bacteriol. 1994;176:3460–3465. doi: 10.1128/jb.176.12.3460-3465.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ninfa A J, Reitzer L J, Magasanik B. Cell. 1987;50:1039–1046. doi: 10.1016/0092-8674(87)90170-x. [DOI] [PubMed] [Google Scholar]
- 7.Buck M, Miller S, Drummond M, Dixon R. Nature (London) 1986;320:374–378. [Google Scholar]
- 8.Merrick M J. Mol Microbiol. 1993;10:903–909. doi: 10.1111/j.1365-2958.1993.tb00961.x. [DOI] [PubMed] [Google Scholar]
- 9.Kustu S, Santero E, Keener J, Popham D, Weiss D. Microbiol Rev. 1989;53:367–376. doi: 10.1128/mr.53.3.367-376.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss D S, Klose K E, Hoover T R, North A K, Porter S C, Wedel A B, Kustu S. In: Transcriptional Regulation. McKnight S L, Yamamoto K R, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1992. pp. 667–694. [Google Scholar]
- 11.Lee J H, Hoover T R. Proc Natl Acad Sci USA. 1995;92:9702–9706. doi: 10.1073/pnas.92.21.9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wedel A, Weiss D S, Popham D, Dröge P, Kustu S. Science. 1990;248:486–489. doi: 10.1126/science.1970441. [DOI] [PubMed] [Google Scholar]
- 13.Merrick M J, Gibbins J, Toukdarian A. Mol Gen Genet. 1987;210:323–330. doi: 10.1007/BF00325701. [DOI] [PubMed] [Google Scholar]
- 14.Wong C, Tintut Y, Gralla J D. J Mol Biol. 1994;236:81–90. doi: 10.1006/jmbi.1994.1120. [DOI] [PubMed] [Google Scholar]
- 15.Cannon W, Missailidis S, Smith C, Cottier A, Austin S, Moore M, Buck M. J Mol Biol. 1995;248:781–803. doi: 10.1006/jmbi.1995.0260. [DOI] [PubMed] [Google Scholar]
- 16.Merrick M J, Coppard J R. Mol Microbiol. 1989;3:1765–1775. doi: 10.1111/j.1365-2958.1989.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 17.Merrick M J, Chambers S. J Bacteriol. 1992;174:7221–7226. doi: 10.1128/jb.174.22.7221-7226.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tintut Y, Wong C, Ying J, Hsieh M, Gralla J D. Proc Natl Acad Sci USA. 1994;91:2120–2124. doi: 10.1073/pnas.91.6.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cullen P J, Foster-Hartnett D, Gabbert K K, Kranz R G. Mol Microbiol. 1994;11:51–65. doi: 10.1111/j.1365-2958.1994.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 20.Buck M, Cannon W. Nature (London) 1992;358:422–424. doi: 10.1038/358422a0. [DOI] [PubMed] [Google Scholar]
- 21.Cannon W, Claverie-Martin F, Austin S, Buck M. Mol Microbiol. 1994;11:227–236. doi: 10.1111/j.1365-2958.1994.tb00303.x. [DOI] [PubMed] [Google Scholar]
- 22.Cannon W, Austin S, Moore M, Buck M. Nucleic Acids Res. 1995;23:351–356. doi: 10.1093/nar/23.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong C, Gralla J D. J Biol Chem. 1992;267:24762–24768. [PubMed] [Google Scholar]
- 24.Merrick M J, Gibbins J R. Nucleic Acids Res. 1985;13:7607–7620. doi: 10.1093/nar/13.21.7607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cannon W, Claverie-Martin F, Austin S, Buck M. Mol Microbiol. 1993;8:287–298. doi: 10.1111/j.1365-2958.1993.tb01573.x. [DOI] [PubMed] [Google Scholar]
- 26.Morett E, Buck M. J Mol Biol. 1989;210:65–77. doi: 10.1016/0022-2836(89)90291-x. [DOI] [PubMed] [Google Scholar]
- 27.Buck M, Cannon W. Mol Microbiol. 1992;6:1625–1630. doi: 10.1111/j.1365-2958.1992.tb00887.x. [DOI] [PubMed] [Google Scholar]
- 28.Maxam A, Gilbert W. Methods Enzymol. 1980;65:499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- 29.Sasse-Dwight S, Gralla J D. Cell. 1990;62:945–954. doi: 10.1016/0092-8674(90)90269-k. [DOI] [PubMed] [Google Scholar]
- 30.Morris L, Cannon W, Claverie-Martin F, Austin S, Buck M. J Biol Chem. 1994;269:11563–11571. [PubMed] [Google Scholar]
- 31.Helmann J D, Chamberlin M. Annu Rev Biochem. 1988;57:839–872. doi: 10.1146/annurev.bi.57.070188.004203. [DOI] [PubMed] [Google Scholar]
- 32.Lonetto M, Gribskov M, Gross C A. J Bacteriol. 1992;174:3843–3849. doi: 10.1128/jb.174.12.3843-3849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tintut Y, Wang J, Gralla J D. Genes Dev. 1995;9:2305–2313. doi: 10.1101/gad.9.18.2305. [DOI] [PubMed] [Google Scholar]
- 34.Wang T J, Syed A, Hsieh M, Gralla J D. Science. 1995;270:992–994. doi: 10.1126/science.270.5238.992. [DOI] [PubMed] [Google Scholar]
- 35.Hsieh M, Gralla J D. J Mol Biol. 1994;239:15–24. doi: 10.1006/jmbi.1994.1347. [DOI] [PubMed] [Google Scholar]
- 36.Hsieh M, Tintut Y, Gralla J D. J Biol Chem. 1994;269:373–378. [PubMed] [Google Scholar]
- 37.Collado-Vides J, Magasanik B, Gralla J. Microbiol Rev. 1991;55:371–394. doi: 10.1128/mr.55.3.371-394.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Popham D L, Szeto D, Keener J, Kustu S. Science. 1989;243:629–635. doi: 10.1126/science.2563595. [DOI] [PubMed] [Google Scholar]
- 39.Malhotra A, Severinova E, Darst S A. Cell. 1996;87:127–136. doi: 10.1016/s0092-8674(00)81329-x. [DOI] [PubMed] [Google Scholar]