Abstract
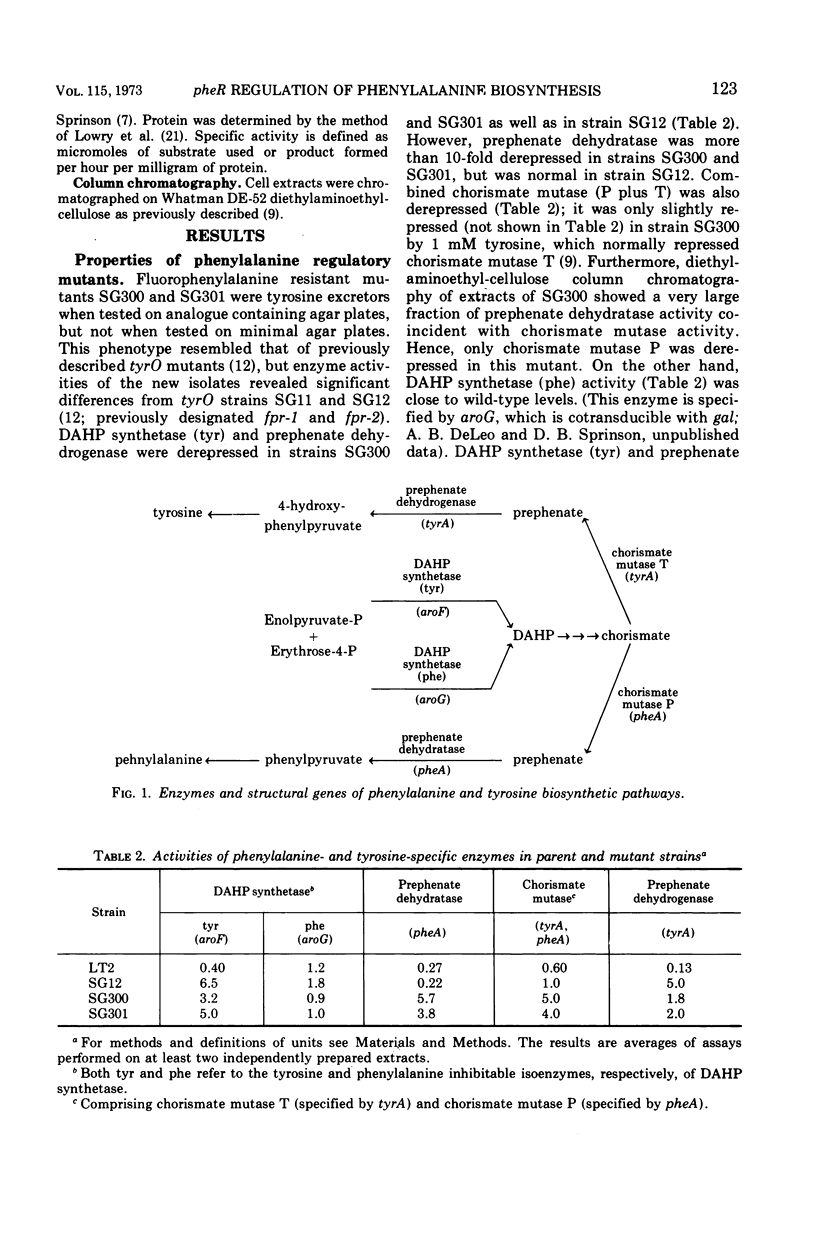
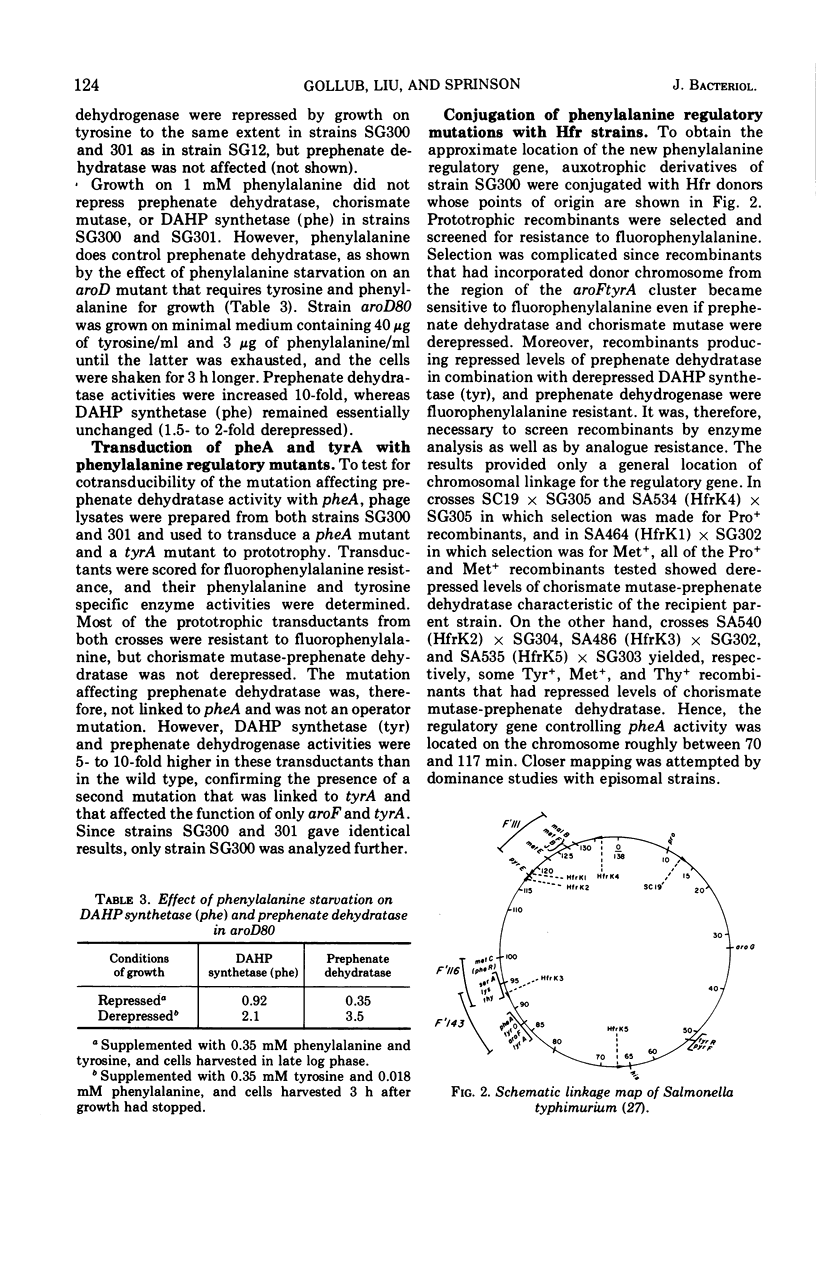
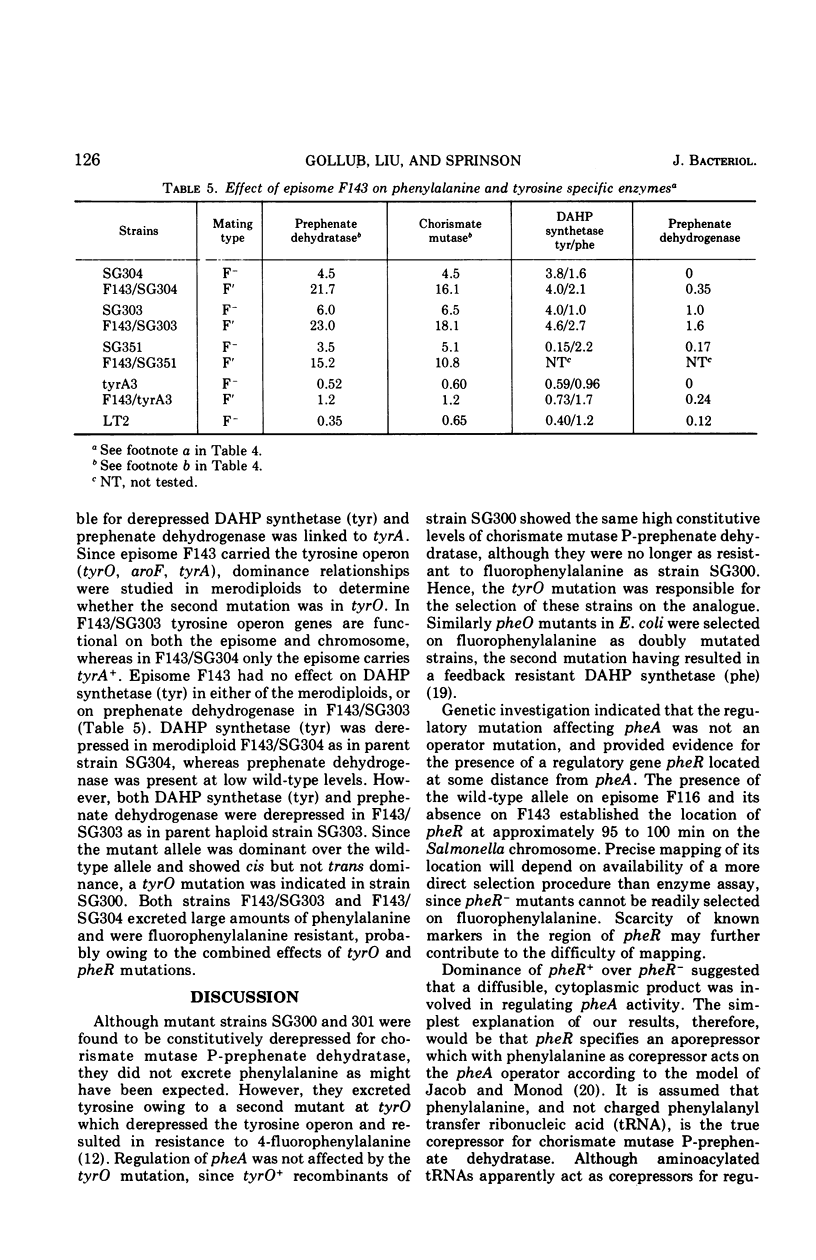
4-Fluorophenylalanine-resistant mutants of Salmonella typhimurium were isolated in which synthesis of chorismate mutase P-prephenate dehydratase (specified by pheA) was highly elevated. Transduction analysis showed that the mutation affecting pheA activity was not linked to pheA, and conjugation and merodiploid analysis indicated that it was in the 95- to 100-min region of the Salmonella chromosome. Evidence is presented for the hypothesis that the mutation responsible for constitutivity of chorismate mutase P-prephenate dehydratase occurred in pheR, a gene specifying a cytoplasmic product that affected pheA. pheR mutants were found to carry a second mutation, tyrO. The tyrO mutation acts cis to cause increased levels of the tyrosine biosynthetic enzymes 3-deoxy-d-arabinoheptulosonate 7-phosphate synthetase (tyr) and prephenate dehydrogenase, but it has no effect on regulation of pheA.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown K. D., Somerville R. L. Repression of aromatic amino acid biosynthesis in Escherichia coli K-12. J Bacteriol. 1971 Oct;108(1):386–399. doi: 10.1128/jb.108.1.386-399.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G., JACOB F. Sur la répression de la synthèse des enzymes intervenant dans la formation du tryptophane chez Escherichia coll. C R Hebd Seances Acad Sci. 1959 Jun 15;248(24):3490–3492. [PubMed] [Google Scholar]
- COTTON R. G., GIBSON F. THE BIOSYNTHESIS OF PHENYLALANINE AND TYROSINE; ENZYMES CONVERTING CHORISMIC ACID INTO PREPHENIC ACID AND THEIR RELATIONSHIPS TO PREPHENATE DEHYDRATASE AND PREPHENATE DEHYDROGENASE. Biochim Biophys Acta. 1965 Apr 12;100:76–88. doi: 10.1016/0304-4165(65)90429-0. [DOI] [PubMed] [Google Scholar]
- Dayan J., Sprinson D. B. Enzyme alterations in tyrosine and phenylalanine auxotrophs of Salmonella typhimurium. J Bacteriol. 1971 Dec;108(3):1174–1180. doi: 10.1128/jb.108.3.1174-1180.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeo A. B., Dayan J., Sprinson D. B. Purification and kinetics of tyrosine-sensitive 3-deoxy-D-arabino-heptulosonic acid 7-phosphate synthetase from Salmonella. J Biol Chem. 1973 Apr 10;248(7):2344–2353. [PubMed] [Google Scholar]
- Fink G. R., Roth J. R. Histidine regulatory mutants in Salmonella typhiumium. VI. Dominance studies. J Mol Biol. 1968 May 14;33(3):547–557. doi: 10.1016/0022-2836(68)90305-7. [DOI] [PubMed] [Google Scholar]
- Gollub E., Sprinson D. B. A regulatory mutation in tyrosine biosynthesis. Biochem Biophys Res Commun. 1969 May 8;35(3):389–395. doi: 10.1016/0006-291x(69)90511-7. [DOI] [PubMed] [Google Scholar]
- Gollub E., Zalkin H., Sprinson D. B. Correlation of genes and enzymes, and studies on regulation of the aromatic pathway in Salmonella. J Biol Chem. 1967 Nov 25;242(22):5323–5328. [PubMed] [Google Scholar]
- HARTMAN P. E., LOPER J. C., SERMAN D. Fine structure mapping by complete transduction between histidine-requiring Salmonella mutants. J Gen Microbiol. 1960 Apr;22:323–353. doi: 10.1099/00221287-22-2-323. [DOI] [PubMed] [Google Scholar]
- Heinonen J., Artz S. W., Zalkin H. Regulation of the tyrosine biosynthetic enzymes in Salmonella typhimurium: analysis of the involvement of tyrosyl-transfer ribonucleic acid and tyrosyl-transfer ribonucleic acid synthetase. J Bacteriol. 1972 Dec;112(3):1254–1263. doi: 10.1128/jb.112.3.1254-1263.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im S. W., Davidson H., Pittard J. Phenylalanine and tyrosine biosynthesis in Escherichia coli K-12: mutants derepressed for 3-deoxy-D-arabinoheptulosonic acid 7-phosphate synthetase (phe), 3-deoxy-D-arabinoheptulosonic acid 7-phosphate synthetase (tyr), chorismate mutase T-prephenate dehydrogenase, and transaminase A. J Bacteriol. 1971 Oct;108(1):400–409. doi: 10.1128/jb.108.1.400-409.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im S. W., Pittard J. Phenylalanine biosynthesis in Escherichia coli K-12: mutants derepressed for chorismate mutase P-prephenate dehydratase. J Bacteriol. 1971 Jun;106(3):784–790. doi: 10.1128/jb.106.3.784-790.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mosteller R. D., Yanofsky C. Evidence that tryptophanyl transfer ribonucleic acid is not the corepressor of the tryptophan operon of Escherichia coli. J Bacteriol. 1971 Jan;105(1):268–275. doi: 10.1128/jb.105.1.268-275.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C. Roles of amino acid activating enzymes in cellular physiology. Bacteriol Rev. 1966 Dec;30(4):701–719. doi: 10.1128/br.30.4.701-719.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka Y., Demerec M., Eisenstark A. Genetic analysis of aromatic mutants of Salmonella typhimurium. Genetics. 1967 Jun;56(2):341–351. doi: 10.1093/genetics/56.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravel J. M., White M. N., Shive W. Activation of tyrosine analogs in relation to enzyme repression. Biochem Biophys Res Commun. 1965 Jul 26;20(3):352–359. doi: 10.1016/0006-291x(65)90372-4. [DOI] [PubMed] [Google Scholar]
- SANDERSON K. E., DEMEREC M. THE LINKAGE MAP OF SALMONELLA TYPHIMURIUM. Genetics. 1965 Jun;51:897–913. doi: 10.1093/genetics/51.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E. Current linkage map of Salmonella typhimurium. Bacteriol Rev. 1970 Jun;34(2):176–193. doi: 10.1128/br.34.2.176-193.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger S., Nester E. W. Mutants of Escherichia coli with an altered tyrosyl-transfer ribonucleic acid synthetase. J Bacteriol. 1969 Oct;100(1):167–175. doi: 10.1128/jb.100.1.167-175.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Levine M. A phage P22 gene controlling integration of prophage. Virology. 1967 Feb;31(2):207–216. doi: 10.1016/0042-6822(67)90164-x. [DOI] [PubMed] [Google Scholar]
- Somerville R. L. Tryptophan operon of Escherichia coli: regulatory behavior in Salmonella typhimurium cytoplasm. Science. 1966 Dec 23;154(3756):1585–1587. doi: 10.1126/science.154.3756.1585. [DOI] [PubMed] [Google Scholar]
- Wallace B. J., Pittard J. Regulator gene controlling enzymes concerned in tyrosine biosynthesis in Escherichia coli. J Bacteriol. 1969 Mar;97(3):1234–1241. doi: 10.1128/jb.97.3.1234-1241.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


