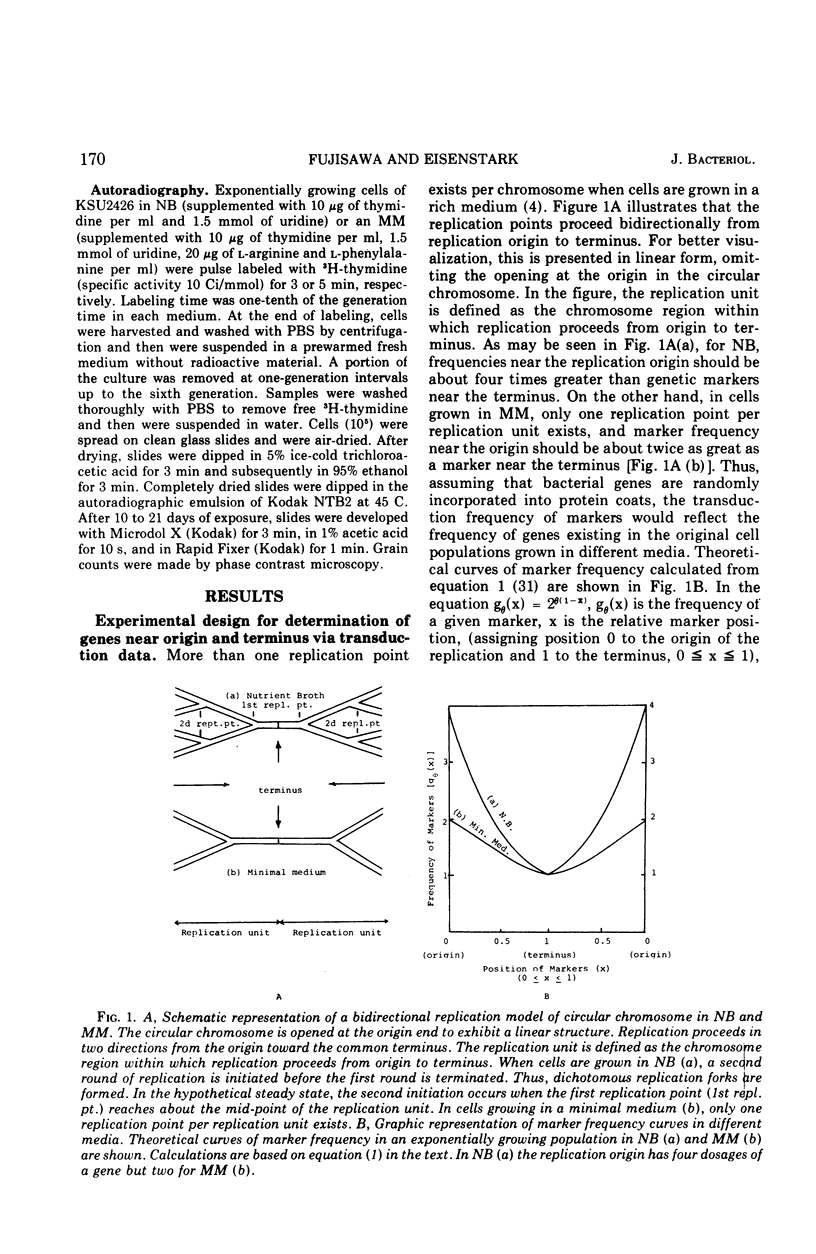
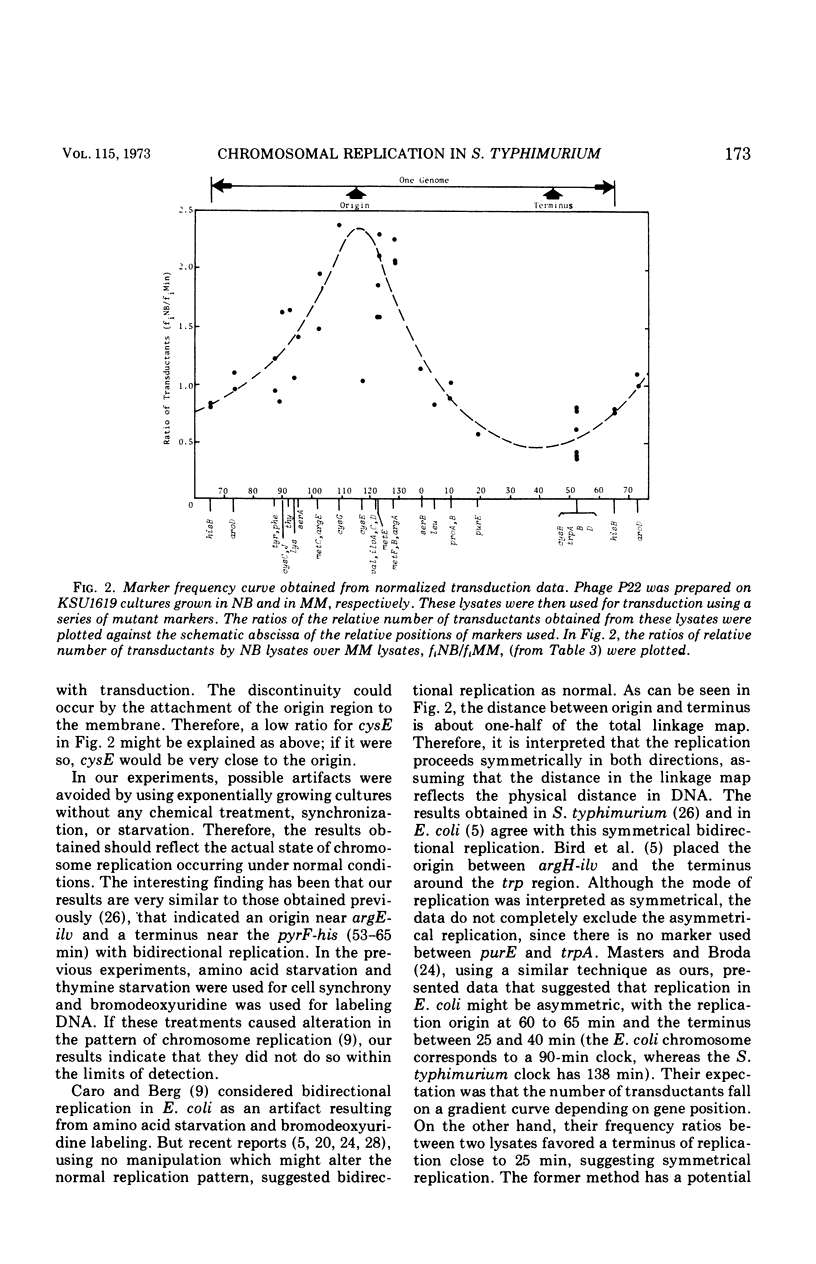
Abstract
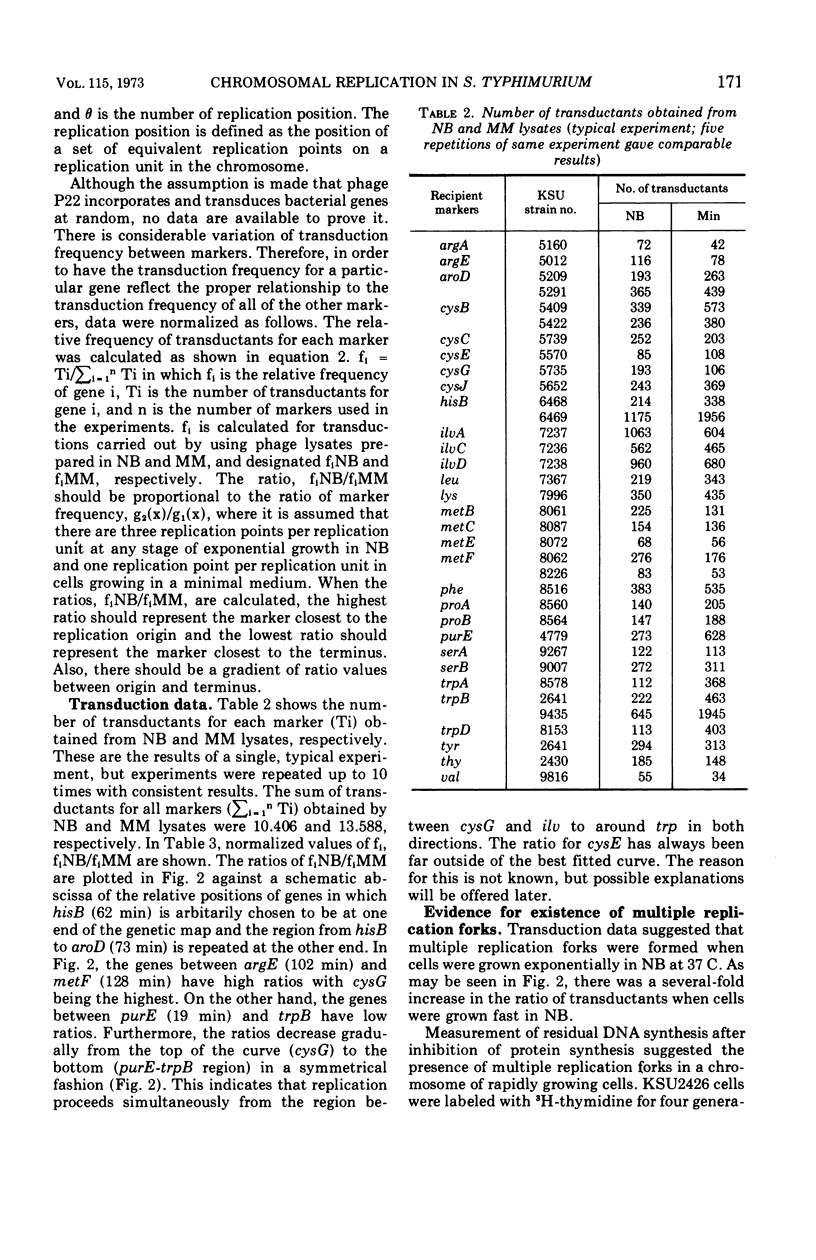
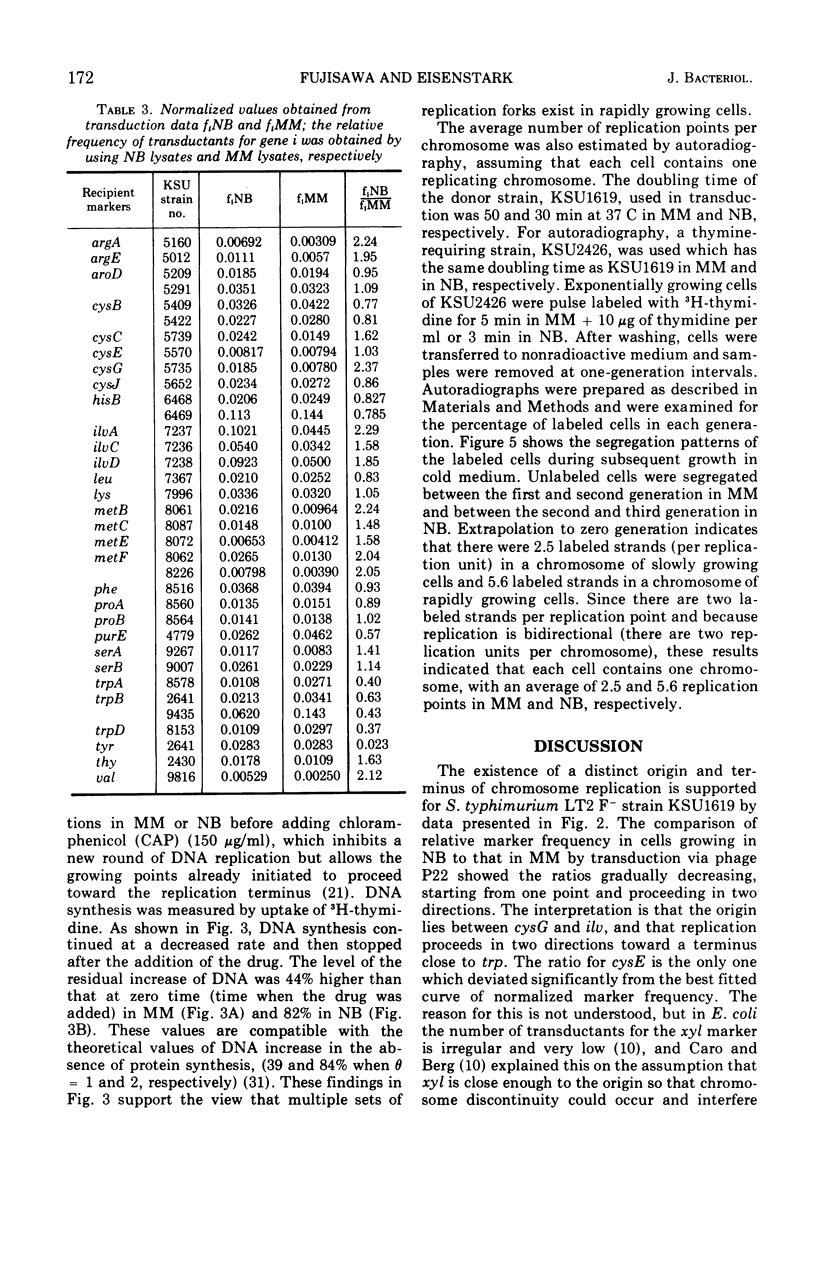
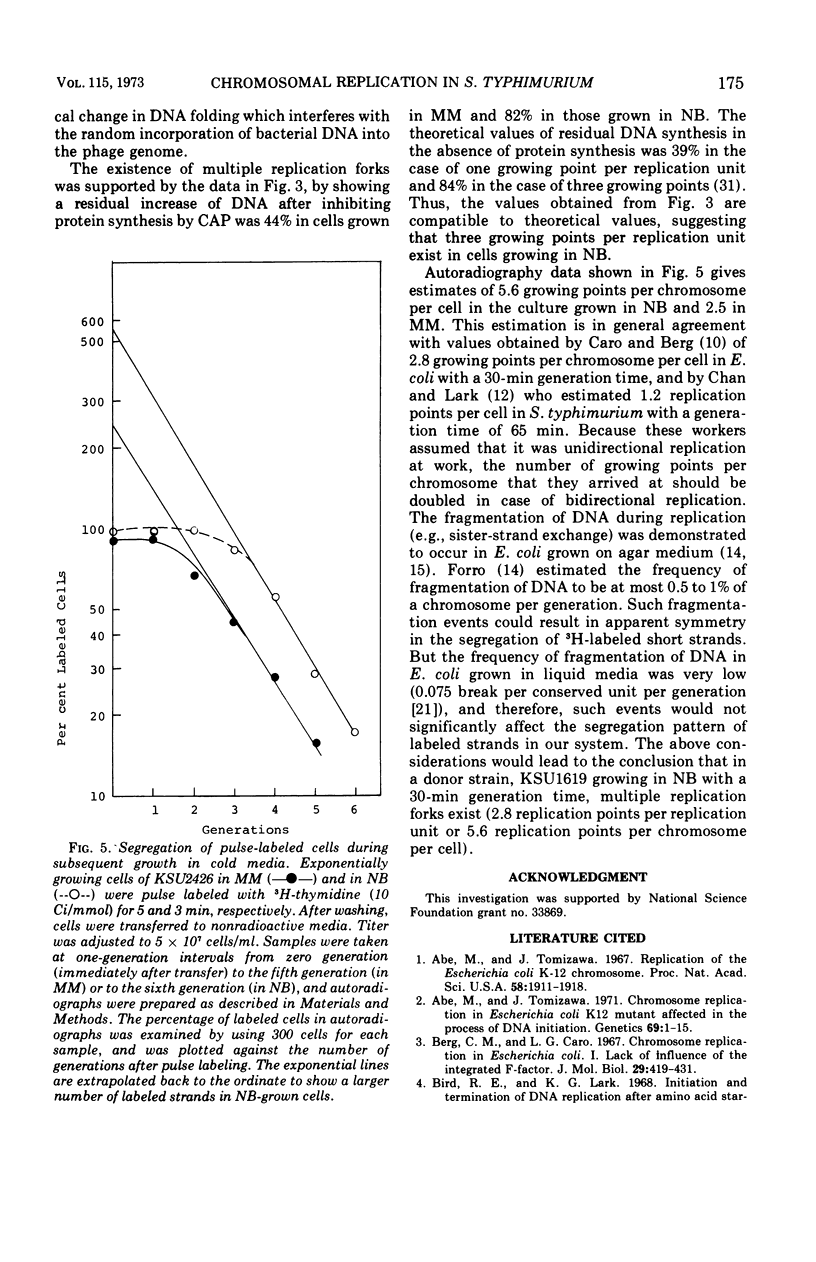
Transducing frequencies of phage P22 lysates prepared from Salmonella typhimurium exponential cultures in minimal and nutrient broth media were compared. The assumption is that cells grown in a minimal medium will have one replication fork per replication unit, but cells in nutrient broth will have multiple replication forks; therefore, the frequency of genetic markers near the origin of replication will be higher in the nutrient broth culture. Analysis of transduction showed a gradient of marker frequencies from the highest (the cysG-ilv region) to the lowest (purE-trpB region) in both clockwise and counter clockwise directions. This supports our previous observation that chromosome replication proceeds bidirectionally from the origin between cysG (109 min on S. typhimurium map) and ilv (122 min) to a terminus in purE-trpB region (20 to 53 min). Since this method avoids possible artifacts of other methods, the results are assumed to reflect the sequence of chromosome replication in exponentially growing cells. Evidence for the existence of multiple replication forks in nutrient broth-grown cells was supported by the following: (i) the marker frequency data fitted the assumption of multiple replication fork formation; (ii) residual deoxyribonucleic acid increase after inhibition of protein synthesis to complete a round of chromosome synthesis which was 44% in cells grown in a minimal medium and 82% in those in nutrient broth; (iii) segregation patterns of the 3H-thymidine-labeled chromosome strands during subsequent growth in non-radioactive medium were studied by autoradiography, and the number of replication points per chromosome per cell was estimated as 5.6 for the nutrient broth culture and 2.5 for the minimal medium culture. These data support a model of symmetrical and bidirectional chromosome replication.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe M., Tomizawa J. Chromosome replication in Escherichia coli K12 mutant affected in the process of DNA initiation. Genetics. 1971 Sep;69(1):1–15. doi: 10.1093/genetics/69.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe M., Tomizawa J. Replication of the escherichia coli K12 chromosome. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1911–1918. doi: 10.1073/pnas.58.5.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONHOEFFER F., GIERER A. ON THE GROWTH MECHANISM OF THE BACTERIAL CHROMOSOME. J Mol Biol. 1963 Nov;7:534–540. doi: 10.1016/s0022-2836(63)80100-x. [DOI] [PubMed] [Google Scholar]
- Bird R. E., Louarn J., Martuscelli J., Caro L. Origin and sequence of chromosome replication in Escherichia coli. J Mol Biol. 1972 Oct 14;70(3):549–566. doi: 10.1016/0022-2836(72)90559-1. [DOI] [PubMed] [Google Scholar]
- CAIRNS J. The bacterial chromosome and its manner of replication as seen by autoradiography. J Mol Biol. 1963 Mar;6:208–213. doi: 10.1016/s0022-2836(63)80070-4. [DOI] [PubMed] [Google Scholar]
- Caro L. G., Berg C. M. Chromosome replication in some strains of Escherichia coli K12. Cold Spring Harb Symp Quant Biol. 1968;33:559–573. doi: 10.1101/sqb.1968.033.01.063. [DOI] [PubMed] [Google Scholar]
- Caro L. G. Chromosome replication in Escherichia coli. 3. Segregation of chromosomal strands in multi-forked replication. J Mol Biol. 1970 Mar 14;48(2):329–338. doi: 10.1016/0022-2836(70)90164-6. [DOI] [PubMed] [Google Scholar]
- Caro L., Berg C. M. Chromosome replication in Escherichia coli. II. Origin of replication in F- and F+ strains. J Mol Biol. 1969 Oct 28;45(2):325–336. doi: 10.1016/0022-2836(69)90108-9. [DOI] [PubMed] [Google Scholar]
- Cerdá-Olmedo E., Hanawalt P. C., Guerola N. Mutagenesis of the replication point by nitrosoguanidine: map and pattern of replication of the Escherichia coli chromosome. J Mol Biol. 1968 May 14;33(3):705–719. doi: 10.1016/0022-2836(68)90315-x. [DOI] [PubMed] [Google Scholar]
- Chan H., Lark K. G. Chromosome replication in Salmonella typhimurium. J Bacteriol. 1969 Feb;97(2):848–860. doi: 10.1128/jb.97.2.848-860.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- FORRO F., Jr, WERTHEIMER S. A. The organization and replication of deoxyribonucleic acid in thymine-deficient strains of Escherichia coli. Biochim Biophys Acta. 1960 May 6;40:9–21. doi: 10.1016/0006-3002(60)91310-x. [DOI] [PubMed] [Google Scholar]
- Forro F., Jr Autoradiographic studies of bacterial chromosome replication in amino-acid deficient Escherichia coli 15T-. Biophys J. 1965 Sep;5(5):629–649. doi: 10.1016/S0006-3495(65)86741-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch A., Worcel A. Symmetric multifork chromosome replication in fast-growing Escherichia coli. J Mol Biol. 1971 Jul 14;59(1):207–211. doi: 10.1016/0022-2836(71)90422-0. [DOI] [PubMed] [Google Scholar]
- Helmstetter C. E. DNA synthesis during the division cycle of rapidly growing Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):507–518. doi: 10.1016/0022-2836(68)90424-5. [DOI] [PubMed] [Google Scholar]
- Helmstetter C. E. Origin and sequence of chromosome replication in Escherichia coli B-r. J Bacteriol. 1968 May;95(5):1634–1641. doi: 10.1128/jb.95.5.1634-1641.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman J. A., Riggs A. D. On the mechanism of DNA replication in mammalian chromosomes. J Mol Biol. 1968 Mar 14;32(2):327–341. doi: 10.1016/0022-2836(68)90013-2. [DOI] [PubMed] [Google Scholar]
- Lark C. G., Consigli R., Toliver A. DNA replication in Chinese hamster cells: evidence for a single replication fork per replicon. J Mol Biol. 1971 Jun 28;58(3):873–875. doi: 10.1016/0022-2836(71)90046-5. [DOI] [PubMed] [Google Scholar]
- Lark K. G., Bird R. E. Segregation of the conserved units of DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1965 Nov;54(5):1444–1450. doi: 10.1073/pnas.54.5.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lark K. G. Regulation of chromosome replication and segregation in bacteria. Bacteriol Rev. 1966 Mar;30(1):3–32. doi: 10.1128/br.30.1.3-32.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters M., Broda P. Evidence for the bidirectional replications of the Escherichia coli chromosome. Nat New Biol. 1971 Aug 4;232(31):137–140. doi: 10.1038/newbio232137a0. [DOI] [PubMed] [Google Scholar]
- Meselson M., Stahl F. W. THE REPLICATION OF DNA IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1958 Jul 15;44(7):671–682. doi: 10.1073/pnas.44.7.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka Y., Eisenstark A. Sequence of genes replicated in Salmonella typhimurium as examined by transduction techniques. J Bacteriol. 1970 May;102(2):320–333. doi: 10.1128/jb.102.2.320-333.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pato M. L., Glaser D. A. The origin and direction of replication of the chromosome of Escherichia coli B-r. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1268–1274. doi: 10.1073/pnas.60.4.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott D. M., Kuempel P. L. Bidirectional replication of the chromosome in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2842–2845. doi: 10.1073/pnas.69.10.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E. Current linkage map of Salmonella typhimurium. Bacteriol Rev. 1970 Jun;34(2):176–193. doi: 10.1128/br.34.2.176-193.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M., Worcel A. Reinitiation of chromosome replication in a thermosensitive DNA mutant of Escherichia coli. II. Synchronization of chromosome replication after temperature shifts. J Mol Biol. 1971 Oct 28;61(2):329–342. doi: 10.1016/0022-2836(71)90383-4. [DOI] [PubMed] [Google Scholar]
- Sueoka N., Yoshikawa H. The chromosome of Bacillus subtilis. I. Theory of marker frequency analysis. Genetics. 1965 Oct;52(4):747–757. doi: 10.1093/genetics/52.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake R. G. Visualization of reinitiated chromosomes in Bacillus subtilis. J Mol Biol. 1972 Jul 28;68(3):501–509. doi: 10.1016/0022-2836(72)90102-7. [DOI] [PubMed] [Google Scholar]
- Ward C. B., Glaser D. A. Origin and direction of DNA synthesis in E. coli B-r. Proc Natl Acad Sci U S A. 1969 Mar;62(3):881–886. doi: 10.1073/pnas.62.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf B., Newman A., Glaser D. A. On the origin and direction of replication of the Escherichia coli K12 chromosome. J Mol Biol. 1968 Mar 28;32(3):611–629. doi: 10.1016/0022-2836(68)90346-x. [DOI] [PubMed] [Google Scholar]
- YOSHIKAWA H., O'SULLIVAN A., SUEOKA N. SEQUENTIAL REPLICATION OF THE BACILLUS SUBTILIS CHROMOSOME. 3. REGULATION OF INITIATION. Proc Natl Acad Sci U S A. 1964 Oct;52:973–980. doi: 10.1073/pnas.52.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]



