Abstract
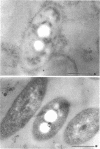
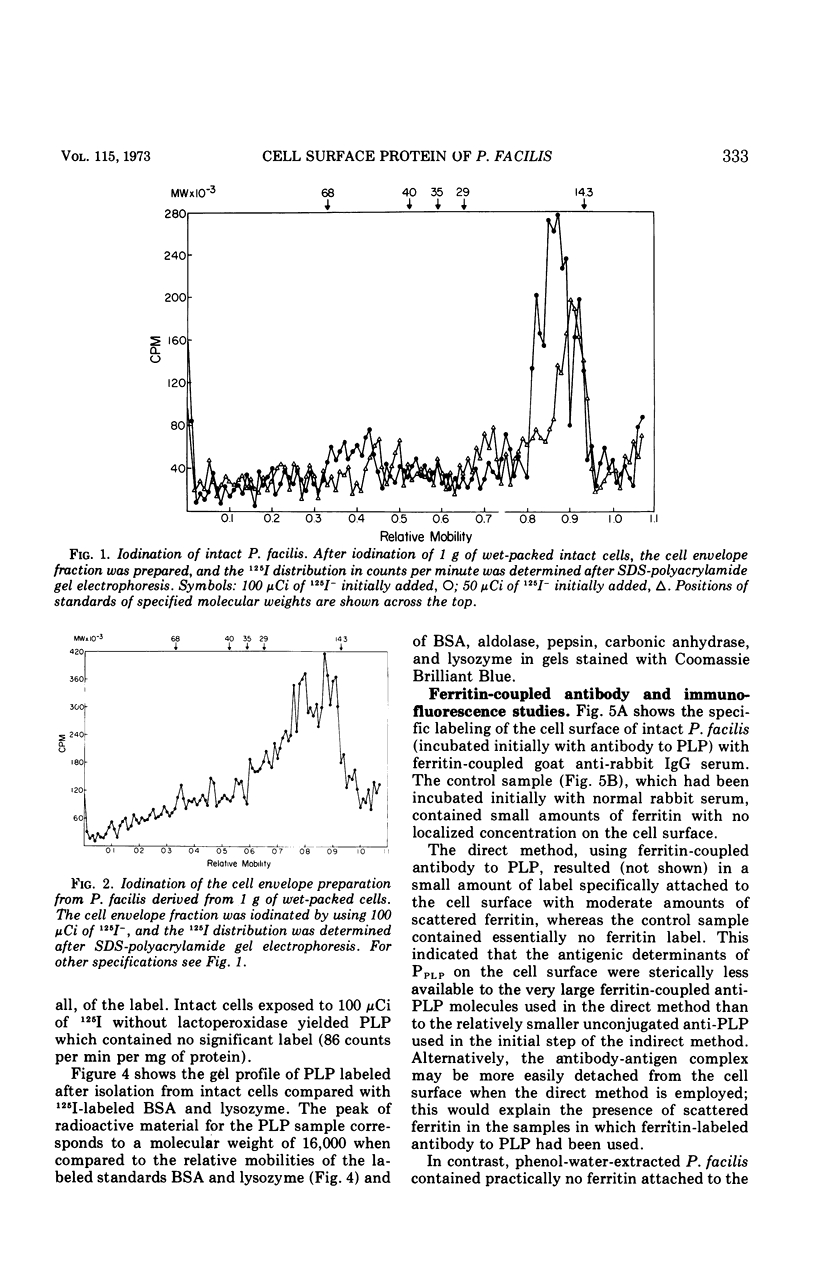
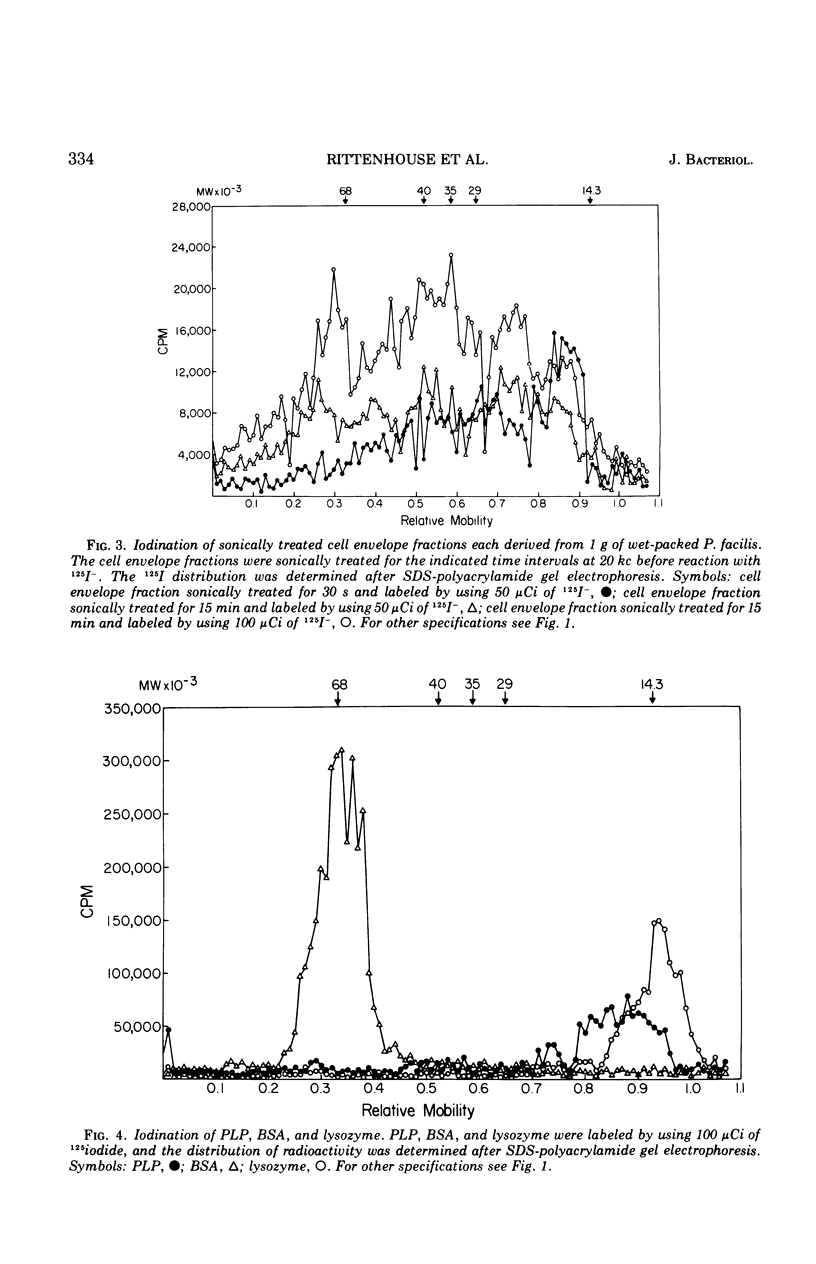
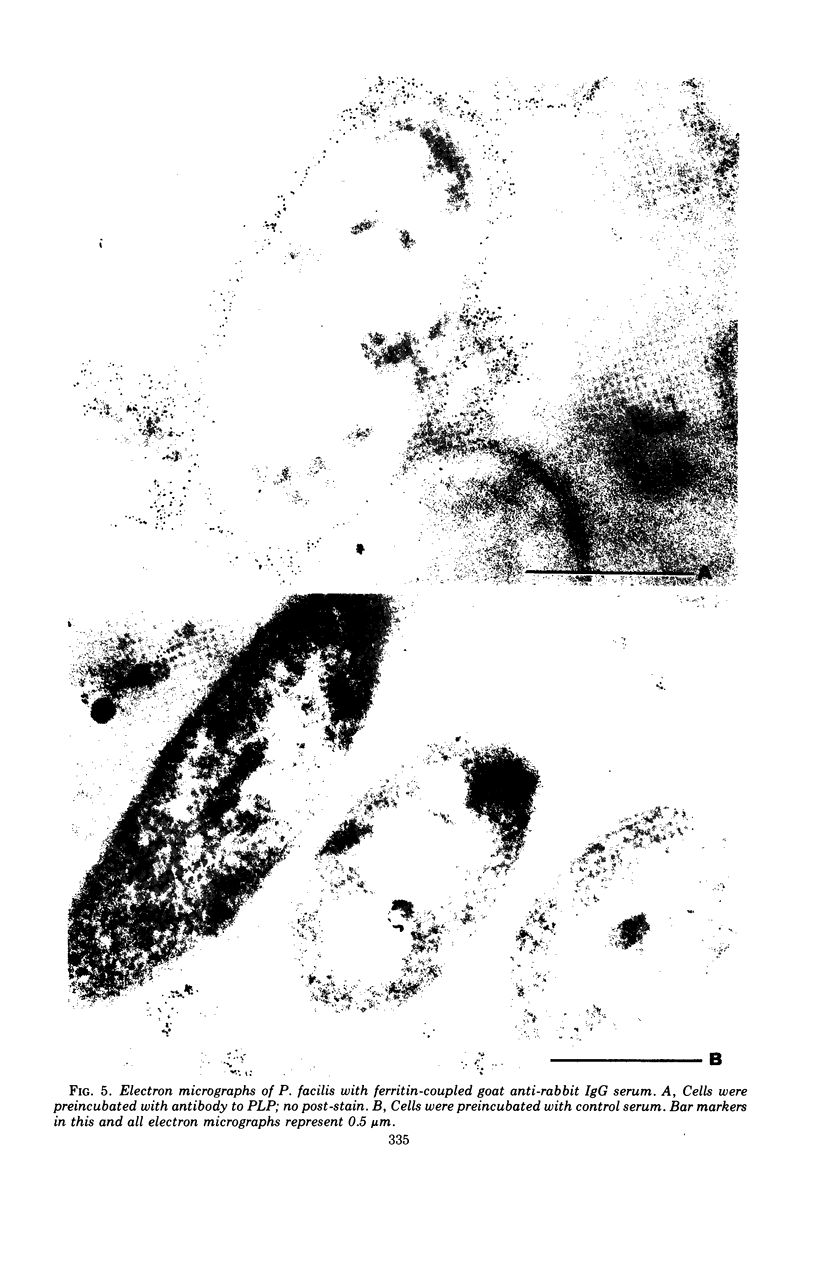
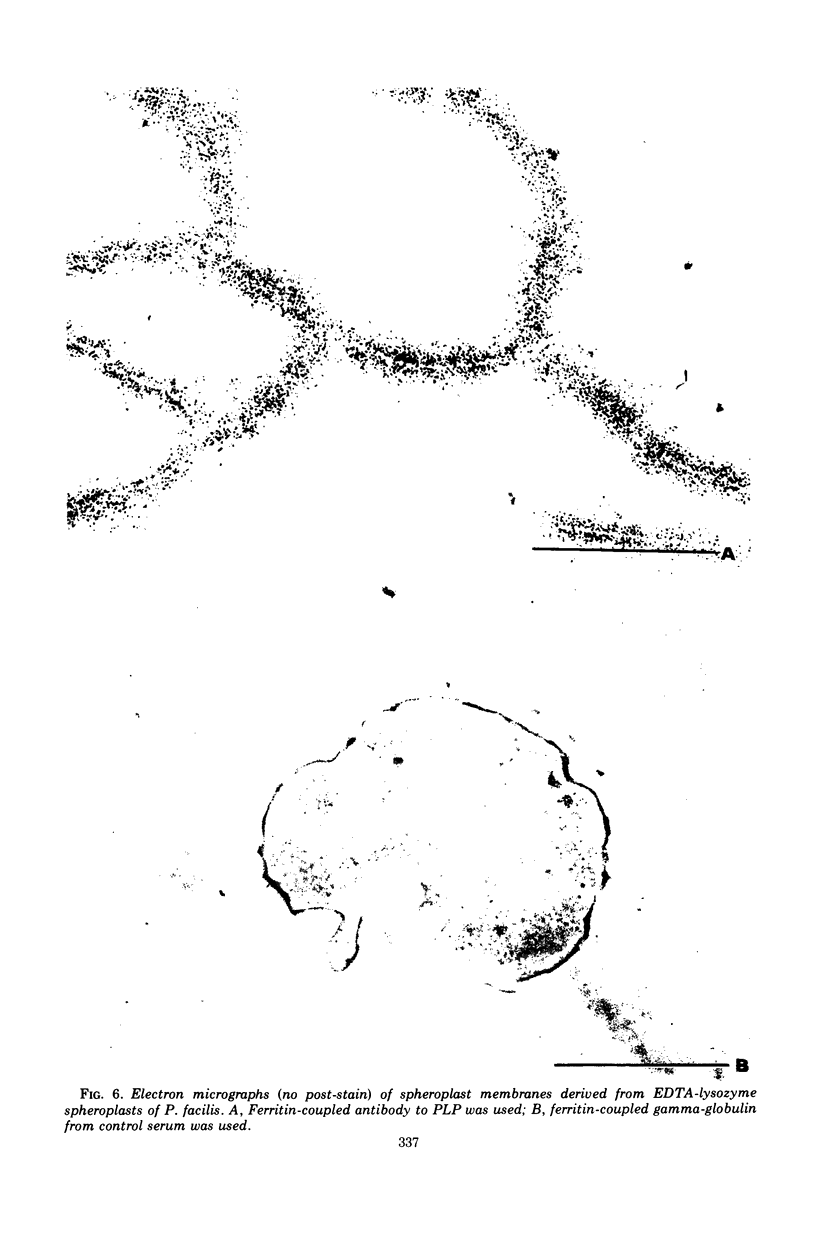
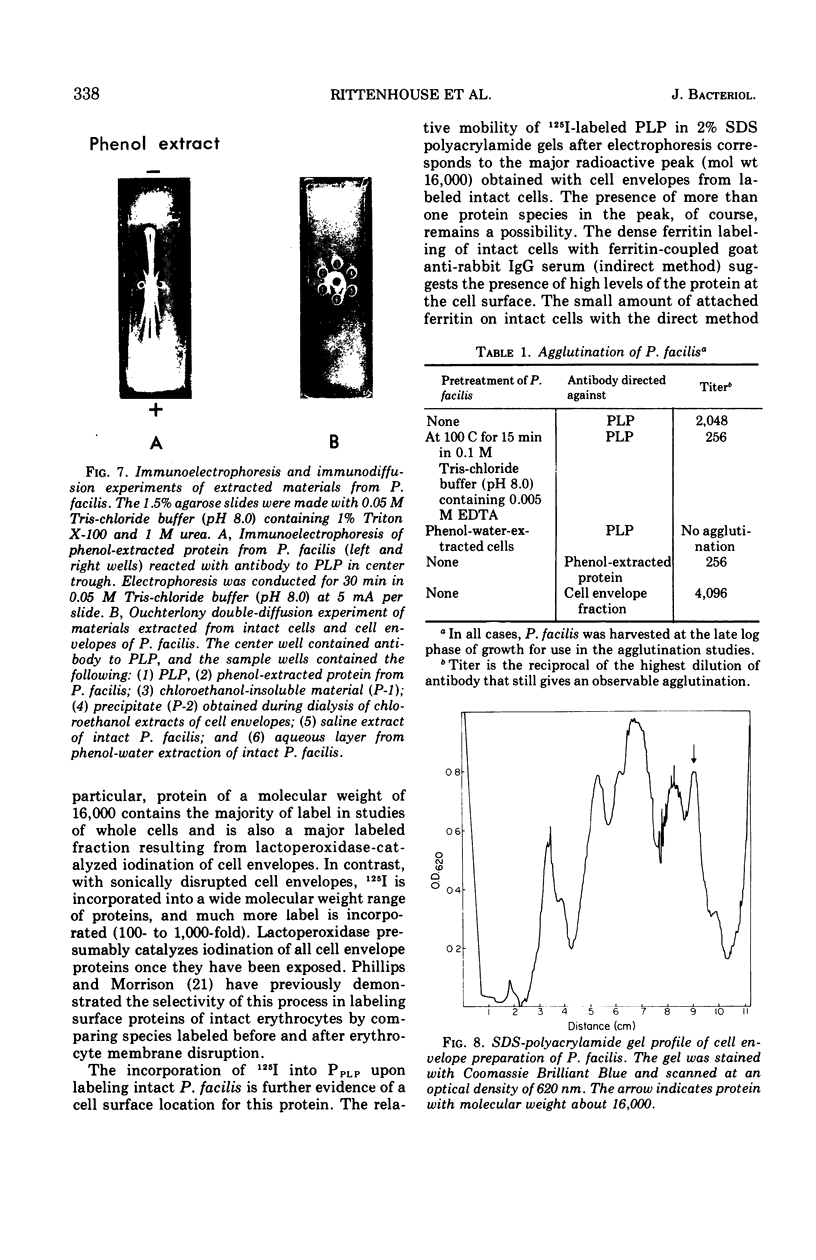
Intact cells of Pseudomonas facilis contain one major molecular weight class of protein that is exposed at the cell surface as revealed by lactoperoxidase-catalyzed iodination with 125I. All molecular weight classes of protein in derived cell envelope preparations are apparently saturated by iodination by lactoperoxidase after prolonged sonic treatment. The molecular weight of the predominantly exposed protein in intact cells is approximately 16,000, which is the minimal molecular weight of a cell envelope protein that precipitates as a complex with phospholipid from extracts of P. facilis. The isolation of labeled phospholipoprotein (PLP) after labeling intact cells with 125I corroborates previous experiments which suggested a surface location for the protein portion of the phospholipoprotein (PPLP). Solvent extraction of cells and immunological evidence, including studies with ferritin-coupled antibodies, indicate that PPLP is located at the cell surface and may also be within the cell envelope. These experiments suggest that PPLP is the major cell surface protein in P. facilis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birdsell D. C., Cota-Robles E. H. Production and ultrastructure of lysozyme and ethylenediaminetetraacetate-lysozyme spheroplasts of Escherichia coli. J Bacteriol. 1967 Jan;93(1):427–437. doi: 10.1128/jb.93.1.427-437.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Organization of proteins in the native and reformed outer membrane of Escherichia coli. Biochim Biophys Acta. 1972 Aug 9;274(2):478–488. doi: 10.1016/0005-2736(72)90193-9. [DOI] [PubMed] [Google Scholar]
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
- Corpe W. A., Salton M. R. Properties of surface materials released from cells of Chromobacterium violaceum. Biochim Biophys Acta. 1966 Jul 27;124(1):125–135. doi: 10.1016/0304-4165(66)90320-5. [DOI] [PubMed] [Google Scholar]
- Cox S. T., Jr, Eagon R. G. Action of ethylenediaminetetraacetic acid, tris(hydroxymethyl)-aminomethane, and lysozyme on cell walls of Pseudomonas aeruginosa. Can J Microbiol. 1968 Aug;14(8):913–922. doi: 10.1139/m68-153. [DOI] [PubMed] [Google Scholar]
- DISCHE Z. Spectrophotometric method for the determination of free pentose and pentose in nucleotides. J Biol Chem. 1949 Nov;181(1):379–392. [PubMed] [Google Scholar]
- De Petris S. Ultrastructure of the cell wall of Escherichia coli and chemical nature of its constituent layers. J Ultrastruct Res. 1967 Jul;19(1):45–83. doi: 10.1016/s0022-5320(67)80059-5. [DOI] [PubMed] [Google Scholar]
- Gray G. W., Wilkinson S. G. The effect of ethylenediaminetetra-acetic acid on the cell walls of some gram-negative bacteria. J Gen Microbiol. 1965 Jun;39(3):385–399. doi: 10.1099/00221287-39-3-385. [DOI] [PubMed] [Google Scholar]
- Heptinstall J., Rittenhouse H. G., McFadden B. A., Shumway L. K. Effect of growth conditions on morphology of Hydrogenomonas facilis and on yield of a phospholipoprotein. J Bacteriol. 1972 Apr;110(1):363–367. doi: 10.1128/jb.110.1.363-367.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Cullen J., Work E. An extracellular lipopolysaccharide-phospholipid-protein complex produced by Escherichia coli grown under lysine-limiting conditions. Biochem J. 1967 Apr;103(1):192–201. doi: 10.1042/bj1030192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn G. D., McFadden B. A. Factors affecting the synthesis and degradation of ribulose-1,5-diphosphate carboxylase in Hydrogenomonas facilis and Hydrogenomonas eutropha. J Bacteriol. 1968 Mar;95(3):937–946. doi: 10.1128/jb.95.3.937-946.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn G. D., McFadden B. A., Johanson R. A., Hill J. M., Shumway L. K. The facile isolation of a structural phospholipoprotein from hydrogenomon as facilis and Neurospora crassa. Proc Natl Acad Sci U S A. 1969 Feb;62(2):407–414. doi: 10.1073/pnas.62.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leive L., Shovlin V. K., Mergenhagen S. E. Physical, chemical, and immunological properties of lipopolysaccharide released from Escherichia coli by ethylenediaminetetraacetate. J Biol Chem. 1968 Dec 25;243(24):6384–6391. [PubMed] [Google Scholar]
- Mergenhagen S. E., Bladen H. A., Hsu K. C. Electron microscopic localization of endotoxic lipopolysaccharide in gram-negative organisms. Ann N Y Acad Sci. 1966 Jun 30;133(2):279–291. doi: 10.1111/j.1749-6632.1966.tb52371.x. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Phillips D. R., Morrison M. Exposed protein on the intact human erythrocyte. Biochemistry. 1971 May 11;10(10):1766–1771. doi: 10.1021/bi00786a006. [DOI] [PubMed] [Google Scholar]
- Poduslo J. F., Greenberg C. S., Glick M. C. Proteins exposed on the surface of mammalian membranes. Biochemistry. 1972 Jul 4;11(14):2616–2621. doi: 10.1021/bi00764a011. [DOI] [PubMed] [Google Scholar]
- Ralston E., Palleroni N. J., Doudoroff M. Deoxyribonucleic acid homologies of some so-called "Hydrogenomonas" species. J Bacteriol. 1972 Jan;109(1):465–466. doi: 10.1128/jb.109.1.465-466.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J. A., Tanford C. The gross conformation of protein-sodium dodecyl sulfate complexes. J Biol Chem. 1970 Oct 10;245(19):5161–5165. [PubMed] [Google Scholar]
- Rittenhouse H. G., Heptinstall J., McFadden B. A. A hydrophobic protein from the cell envelope of Hydrogenomonas facilis. Biochemistry. 1971 Oct 26;10(22):4045–4049. doi: 10.1021/bi00798a006. [DOI] [PubMed] [Google Scholar]
- Rittenhouse H. G., Rodda J. B., McFadden B. A. Immunologically cross-reacting proteins in cell walls of many bacteria. J Bacteriol. 1973 Mar;113(3):1400–1403. doi: 10.1128/jb.113.3.1400-1403.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothfield L., Pearlman-Kothencz M. Synthesis and assembly of bacterial membrane components. A lipopolysaccharide-phospholipid-protein complex excreted by living bacteria. J Mol Biol. 1969 Sep 28;44(3):477–492. doi: 10.1016/0022-2836(69)90374-x. [DOI] [PubMed] [Google Scholar]
- SCHICK A. F., SINGER S. J. On the formation of covalent linkages between two protein molecules. J Biol Chem. 1961 Sep;236:2477–2485. [PubMed] [Google Scholar]
- Schnaitman C. A. Examination of the protein composition of the cell envelope of Escherichia coli by polyacrylamide gel electrophoresis. J Bacteriol. 1970 Nov;104(2):882–889. doi: 10.1128/jb.104.2.882-889.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W., FRANK H., MARTIN H. H. The rigid layer of the cell wall of Escherichia coli strain B. J Gen Microbiol. 1960 Feb;22:158–166. doi: 10.1099/00221287-22-1-158. [DOI] [PubMed] [Google Scholar]
- Weckesser J., Drews G., Ladwig R. Localization and biological and physicochemical properties of the cell wall lipopolysaccharide of Rhodopseudomonas capsulata. J Bacteriol. 1972 Apr;110(1):346–353. doi: 10.1128/jb.110.1.346-353.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S. G. Cell walls of pseudomonas species sensitive to ethylenediaminetetraacetic Acid. J Bacteriol. 1970 Dec;104(3):1035–1044. doi: 10.1128/jb.104.3.1035-1044.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]