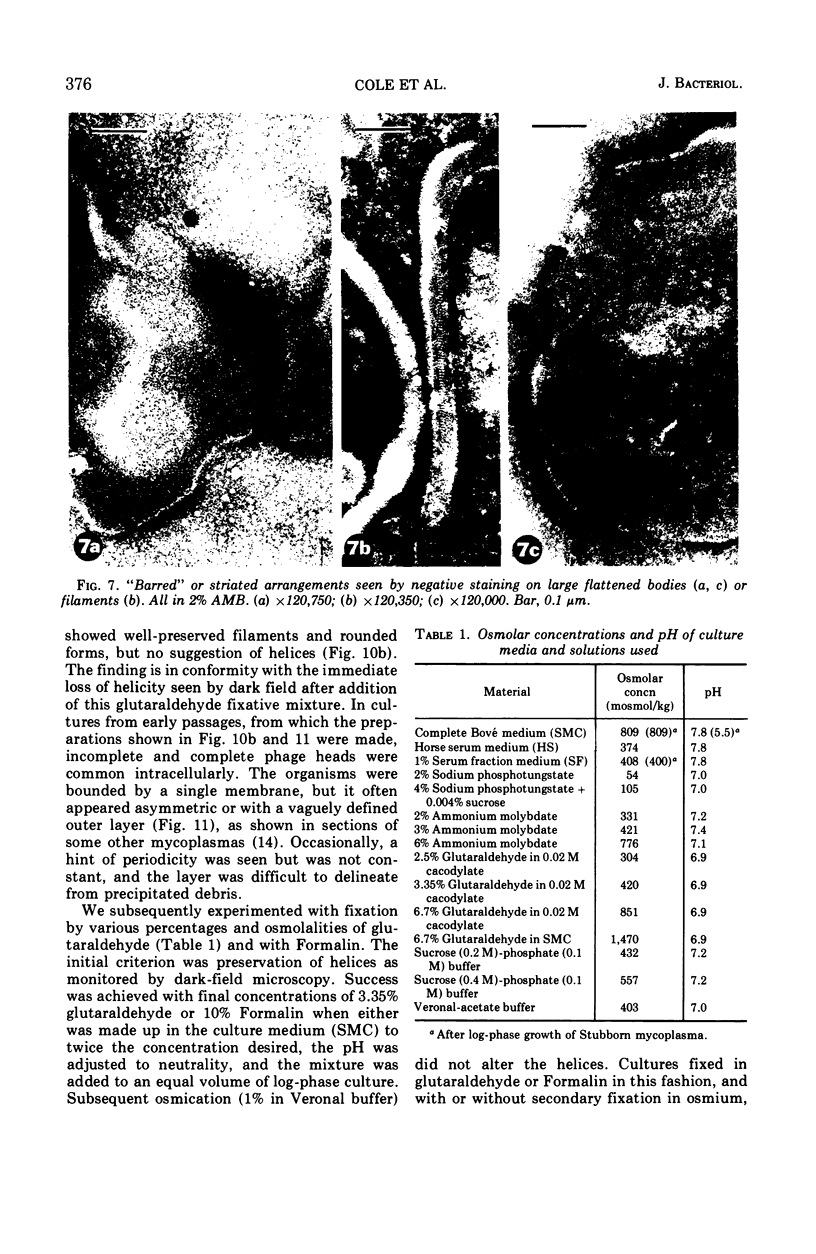
Abstract
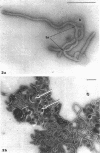
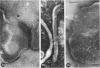
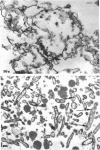
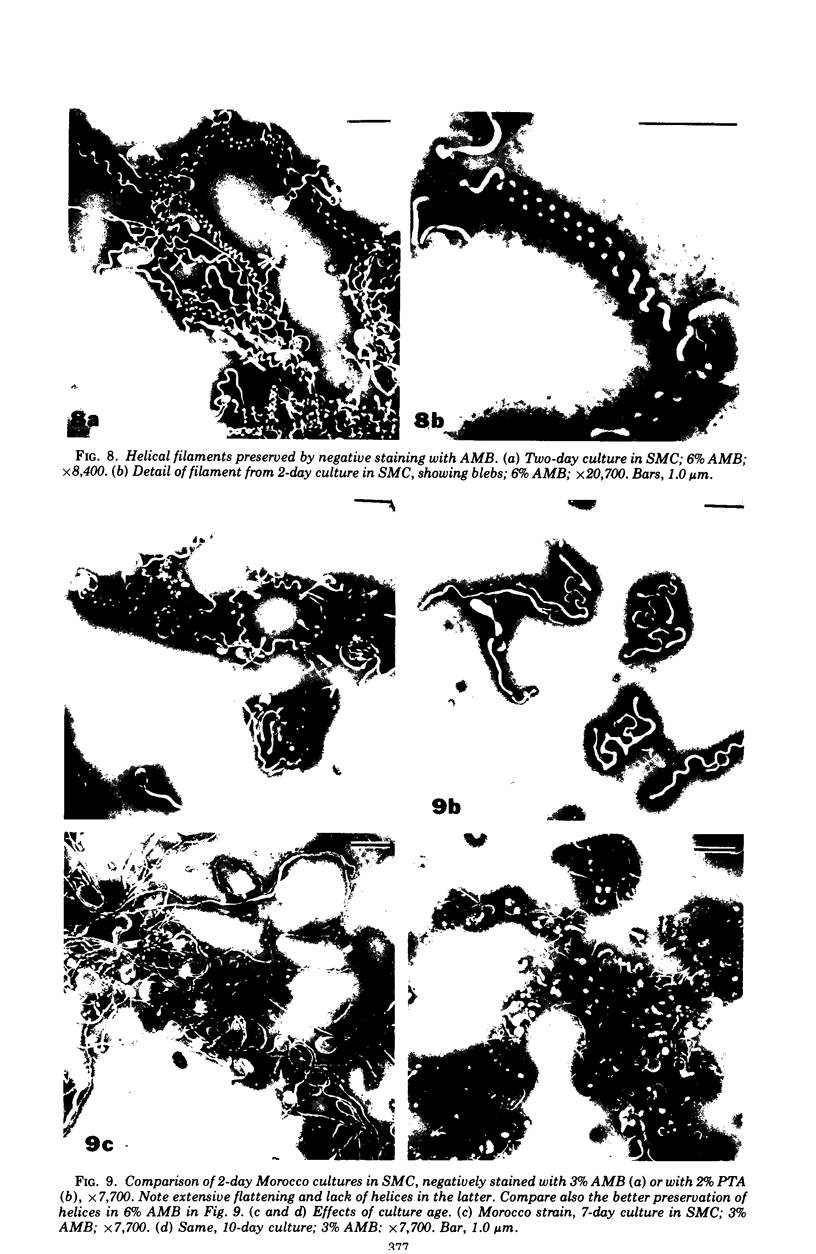
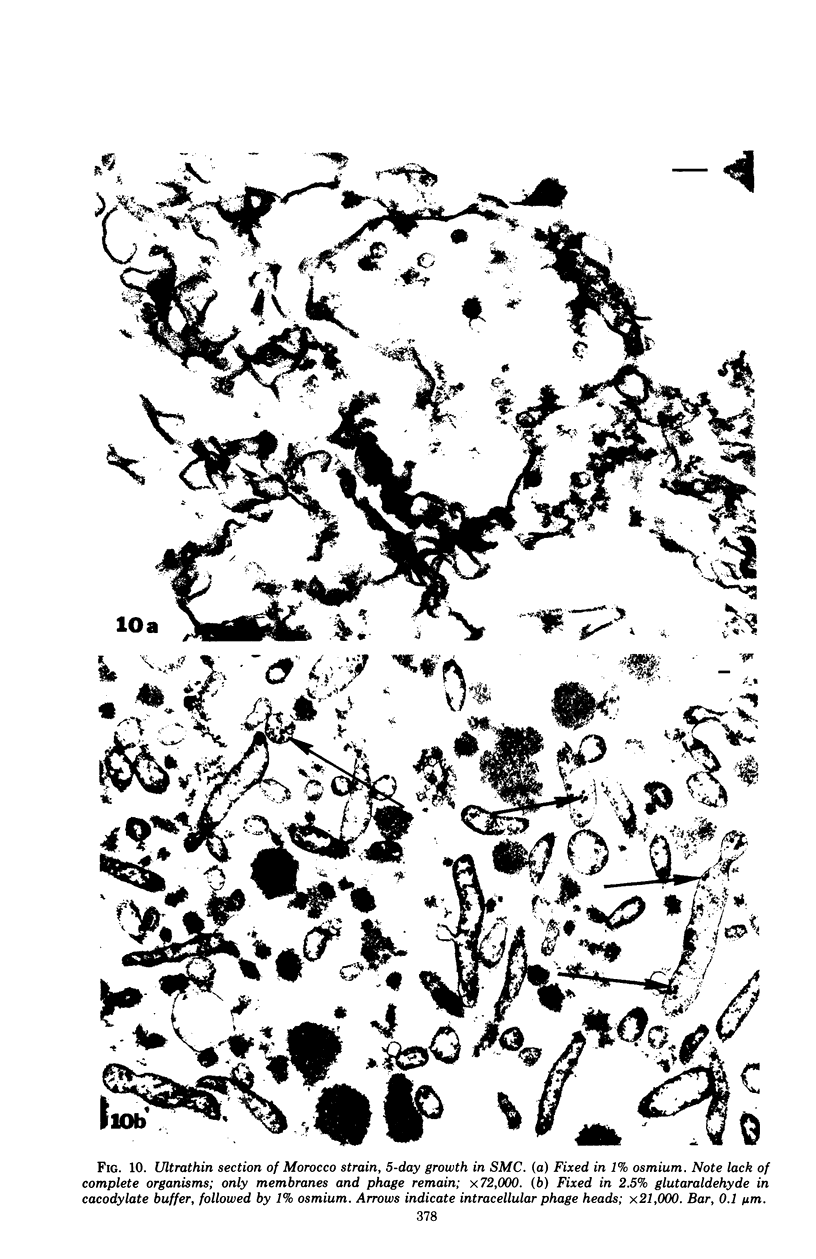
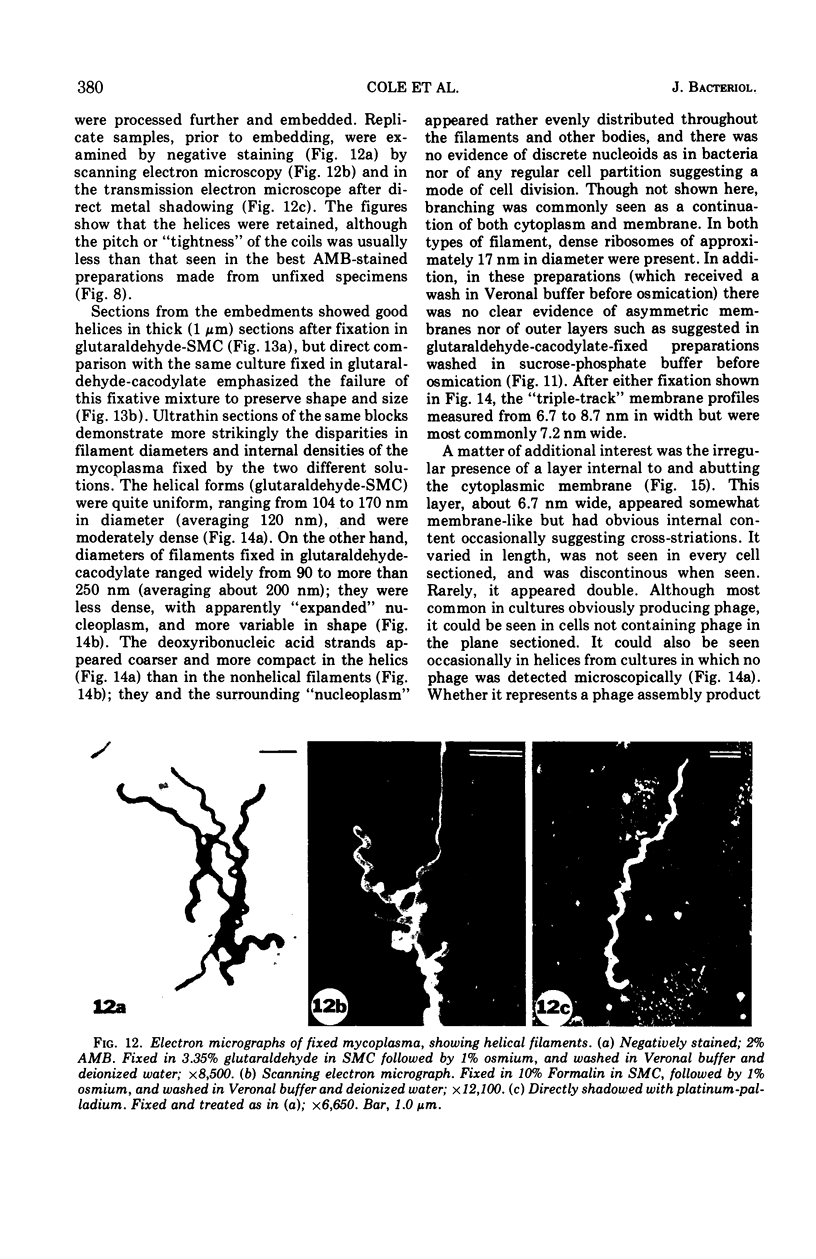
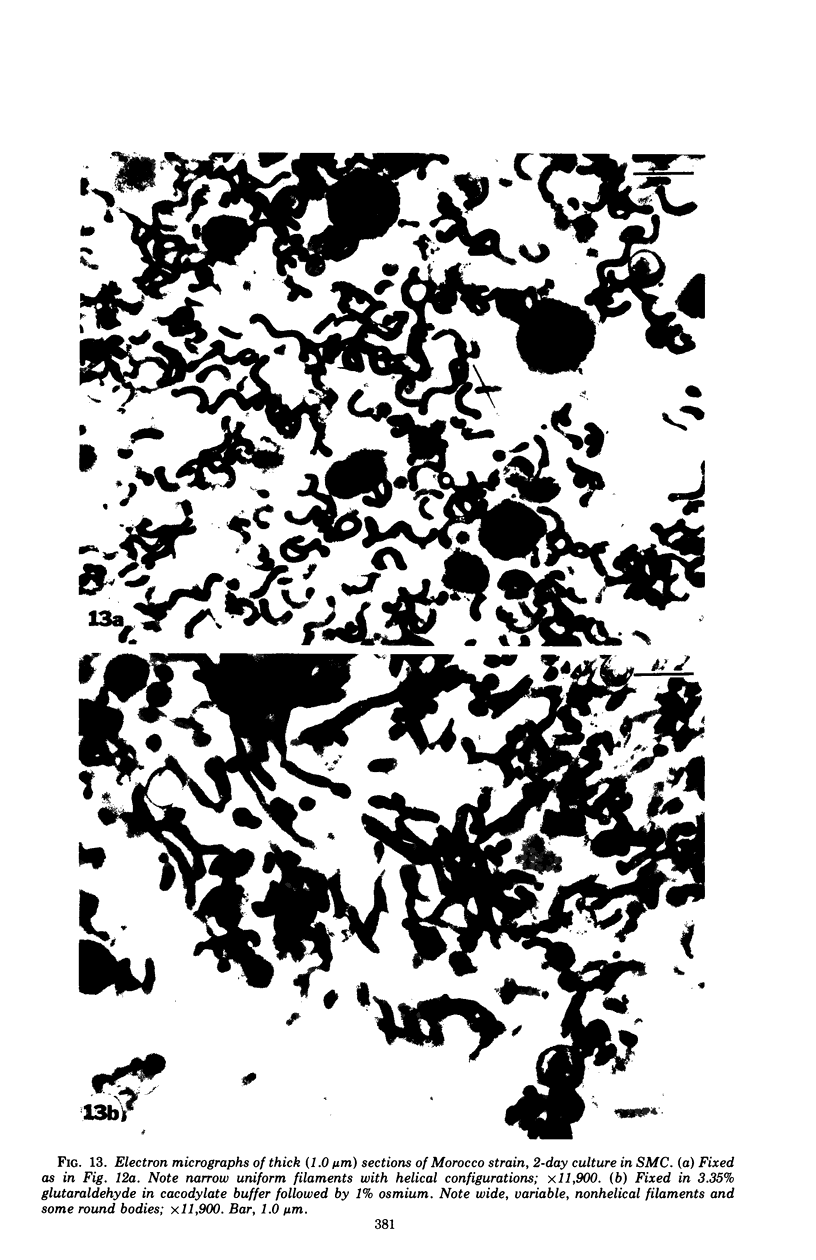
The mycoplasma-like organism Spiroplasma citri gen. nov., sp. nov., isolated from citrus infected with “Stubborn” disease and carried in serial cultures in several media, was examined by dark-field microscopy and electron microscopy of negatively-stained and shadowed preparations and of sections. It grows as motile, helical filaments in liquid, but as nonmotile, nonhelical filaments and round bodies in agar cultures. Helicity and motility are lost in old broth cultures and upon addition of a variety of negative stains, fixatives, and other solutions. No organelles accounting for motility are present, but a layer of surface projections is present on the surface of the single, bounding membrane. The mycoplasma produces a tailed, type B bacteriophage which appear to attach to the outer layer. Helical filaments are preserved in ammonium molybdate, but not in sodium phosphotungstate, and by fixation in Formalin or glutaraldehyde made up in medium, but not by osmium nor by glutaraldehyde in cacodylate buffer. This mycoplasma appears similar to the noncultured helical microorganism in corn stunt-diseased tissues and is probably a representative of a new group of mycoplasmas which are in possession of surface projections, rotary motility, and bacteriophage infection.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen T. C., Stevens J. O., Florance E. R., Hampton R. O. Ultrastructure of Mycoplasma gallisepticum isolate 1056. J Ultrastruct Res. 1970 Nov;33(3):318–331. doi: 10.1016/s0022-5320(70)90025-0. [DOI] [PubMed] [Google Scholar]
- Biberfeld G., Biberfeld P. Ultrastructural features of Mycoplasma pneumoniae. J Bacteriol. 1970 Jun;102(3):855–861. doi: 10.1128/jb.102.3.855-861.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black F. T., Birch-Andersen A., Freundt E. A. Morphology and ultrastructure of human T-mycoplasmas. J Bacteriol. 1972 Jul;111(1):254–259. doi: 10.1128/jb.111.1.254-259.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boatman E. S., Kenny G. E. Morphology and ultrastructure of Mycoplasma pneumoniae spherules. J Bacteriol. 1971 Jun;106(3):1005–1015. doi: 10.1128/jb.106.3.1005-1015.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt W. Celular morphology of newly isolated Mycoplasma hominis strains. J Bacteriol. 1971 Jan;105(1):449–450. doi: 10.1128/jb.105.1.449-450.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt W., Höfling K. H., Heunert H. H., Milthaler B. Messungen an beweglichen Zellen von Mycoplasma pneumoniae. Z Med Mikrobiol Immunol. 1970;156(1):39–43. [PubMed] [Google Scholar]
- Chen T. A., Granados R. R. Plant-Pathogenic Mycoplasma-Like Organism: Maintenance in vitro and Transmission to Zea mays L. Science. 1970 Mar 20;167(3925):1633–1636. doi: 10.1126/science.167.3925.1633. [DOI] [PubMed] [Google Scholar]
- Chu H. P., Horne R. W. Electron microscopy of Mycoplasma gallisepticum and Mycoplasma mycoides using the negative staining technique and their comparison with myxovirus. Ann N Y Acad Sci. 1967 Jul 28;143(1):190–203. doi: 10.1111/j.1749-6632.1967.tb27658.x. [DOI] [PubMed] [Google Scholar]
- Clyde W. A., Jr, Kim K. S. Biophysical characterization of human mycoplasma species. Ann N Y Acad Sci. 1967 Jul 28;143(1):425–435. doi: 10.1111/j.1749-6632.1967.tb27687.x. [DOI] [PubMed] [Google Scholar]
- Cox C. D. Shape of Treponema pallidum. J Bacteriol. 1972 Feb;109(2):943–944. doi: 10.1128/jb.109.2.943-944.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOMERMUTH C. H., NIELSEN M. H., FREUNDT E. A., BIRCH-ANDERSEN A. ULTRASTRUCTURE OF MYCOPLASMA SPECIES. J Bacteriol. 1964 Sep;88:727–744. doi: 10.1128/jb.88.3.727-744.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. E., Worley J. F., Whitcomb R. F., Ishijima T., Steere R. L. Helical filaments produced by a Mycoplasma-like organism associated with corn stunt disease. Science. 1972 May 5;176(4034):521–523. doi: 10.1126/science.176.4034.521. [DOI] [PubMed] [Google Scholar]
- Favre R., Boy de la Tour E., Segrè N., Kellenberger E. Studies on the morphopoiesis of the head of phage T-even. I. Morphological, immunological, and genetic characterization of polyheads. J Ultrastruct Res. 1965 Oct;13(3):318–342. doi: 10.1016/s0022-5320(65)80080-6. [DOI] [PubMed] [Google Scholar]
- Forte T. M., Nichols A. V., Gong E. L., Levy R. I., Lux S. Electron microscopic study on reassembly of plasma high density apoprotein with various lipids. Biochim Biophys Acta. 1971 Nov 5;248(2):381–386. doi: 10.1016/0005-2760(71)90026-9. [DOI] [PubMed] [Google Scholar]
- Furness G. The growth and morphology of Mycoplasmas replicating in synchrony. J Infect Dis. 1970 Sep;122(3):146–158. doi: 10.1093/infdis/122.3.146. [DOI] [PubMed] [Google Scholar]
- Glauert A. M., Lucy J. A. Electron microscopy of lipids: effects of pH and fixatives on the appearance of a macromolecular assembly of lipid micelles in negatively stained preparations. J Microsc. 1969;89(1):1–18. doi: 10.1111/j.1365-2818.1969.tb00645.x. [DOI] [PubMed] [Google Scholar]
- Gourlay R. N., Bruce J., Garwes D. J. Characterization of Mycoplasmatales virus laidlawii 1. Nat New Biol. 1971 Jan 27;229(4):118–119. doi: 10.1038/newbio229118a0. [DOI] [PubMed] [Google Scholar]
- Gourlay R. N. Isolation of a virus infecting a strain of Mycoplasma laidlawii. Nature. 1970 Mar 21;225(5238):1165–1165. doi: 10.1038/2251165a0. [DOI] [PubMed] [Google Scholar]
- Gourlay R. N. Mycoplasmatales virus-laidlawii 2, a new virus isolated from Acholeplasma laidlawii. J Gen Virol. 1971 Jul;12(1):65–67. doi: 10.1099/0022-1317-12-1-65. [DOI] [PubMed] [Google Scholar]
- Granados R. R., Maramorosch K., Shikata E. Mycoplasma: SUSPECTED ETIOLOGIC AGENT OF CORN STUNT. Proc Natl Acad Sci U S A. 1968 Jul;60(3):841–844. doi: 10.1073/pnas.60.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. A., Larson K., Weibull C. The shape of pleuropneumonia-like organisms (PPLO, mycoplasma) in liquid media. Z Allg Mikrobiol. 1967;7(3):233–234. [PubMed] [Google Scholar]
- Kammer G. M., Pollack J. D., Klainer A. S. Scanning-beam electron microscopy of Mycoplasma pneumoniae. J Bacteriol. 1970 Oct;104(1):499–502. doi: 10.1128/jb.104.1.499-502.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemcke R. M. Osmolar concentration and fixation of mycoplasmas. J Bacteriol. 1972 Jun;110(3):1154–1162. doi: 10.1128/jb.110.3.1154-1162.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liss A., Maniloff J. Isolation of Mycoplasmatales viruses and characterization of MVL1, MVL52, and MVG51. Science. 1971 Aug 20;173(3998):725–727. doi: 10.1126/science.173.3998.725. [DOI] [PubMed] [Google Scholar]
- Maniloff J. Ultrastructure of Mycoplasma laidlawii during culture development. J Bacteriol. 1970 May;102(2):561–572. doi: 10.1128/jb.102.2.561-572.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz J., Bredt W. Elektronenmikroskopische Untersuchungen an Mycoplasma hominis (Stamm W 463-69. Z Med Mikrobiol Immunol. 1971;156(4):368–378. [PubMed] [Google Scholar]
- Muscatello U., Horne R. W. Effect of the tonicity of some negative-staining solutions on the elementary structure of membrane-bounded systems. J Ultrastruct Res. 1968 Oct;25(1):73–83. doi: 10.1016/s0022-5320(68)80061-9. [DOI] [PubMed] [Google Scholar]
- Nelson J. B., Lyons M. J. Phase-contrast and electron microscopy of murine strains of Mycoplasma. J Bacteriol. 1965 Dec;90(6):1750–1763. doi: 10.1128/jb.90.6.1750-1763.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K., Poulson D. F. A virus associated with SR-spirochetes of Drosophila nebulosa. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1565–1572. doi: 10.1073/pnas.67.3.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popkin T. J., Theodore T. S., Cole R. M. Electron microscopy during release and purification of mesosomal vesicles and protoplast membranes from Staphylococcus aureus. J Bacteriol. 1971 Sep;107(3):907–917. doi: 10.1128/jb.107.3.907-917.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodwell A. W. The stability of Mycoplasma mycoides. J Gen Microbiol. 1965 Aug;40(2):227–234. doi: 10.1099/00221287-40-2-227. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Woods D. A. Electron microscopic studies of Mycoplasma pulmonis (Negroni strain). J Gen Microbiol. 1970 Nov;63(3):281–287. doi: 10.1099/00221287-63-3-281. [DOI] [PubMed] [Google Scholar]
- Tikhonenko A. S. Izvrashchennyi morfogenez obolochki GOLOVKI FAGA No. 1 Bacillus mycoides. Mikrobiologiia. 1966 Jan-Feb;35(1):118–121. [PubMed] [Google Scholar]
- Wolanski B., Maramorosch K. Negatively stained mycoplasmas: fact or artifact? Virology. 1970 Oct;42(2):319–327. doi: 10.1016/0042-6822(70)90276-x. [DOI] [PubMed] [Google Scholar]
- Zweig M., Rosenkranz H. S., Morgan C. Development of coliphage T5: ultrastructural and biochemical studies. J Virol. 1972 Mar;9(3):526–543. doi: 10.1128/jvi.9.3.526-543.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]