Abstract
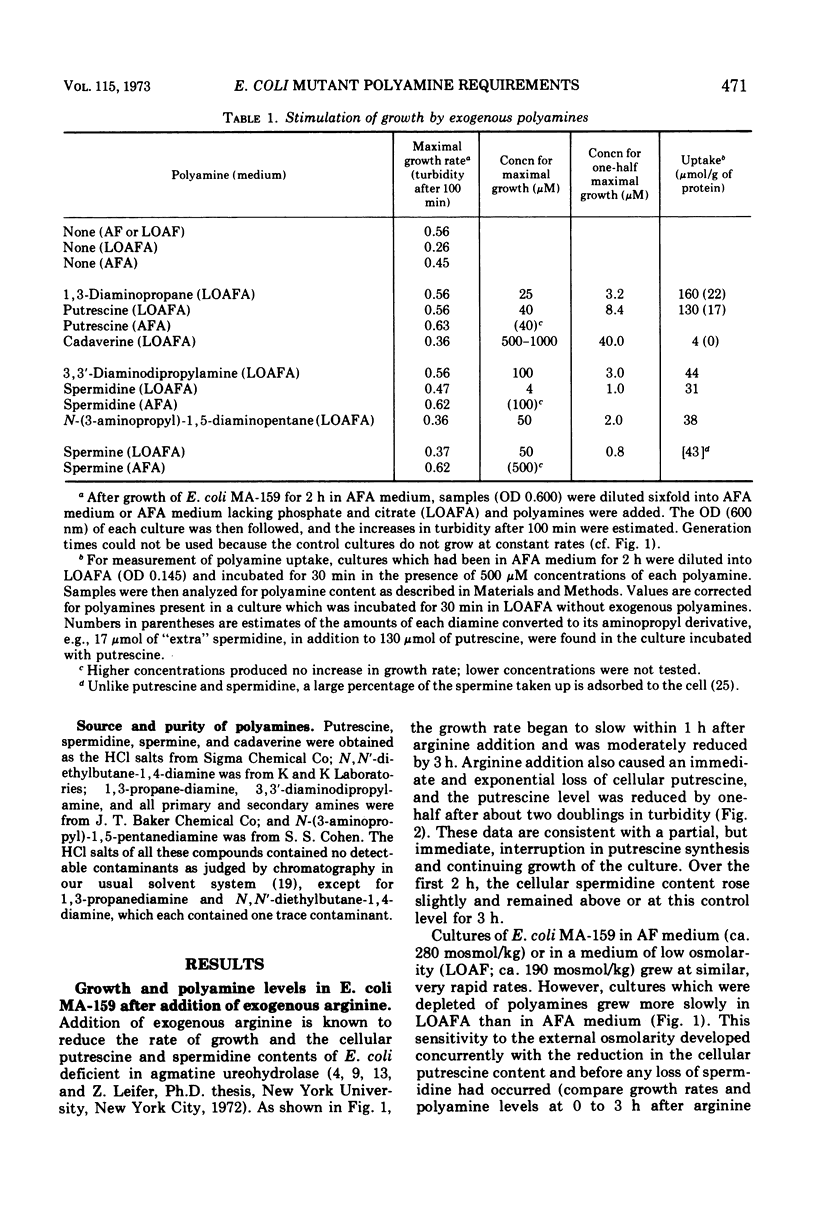
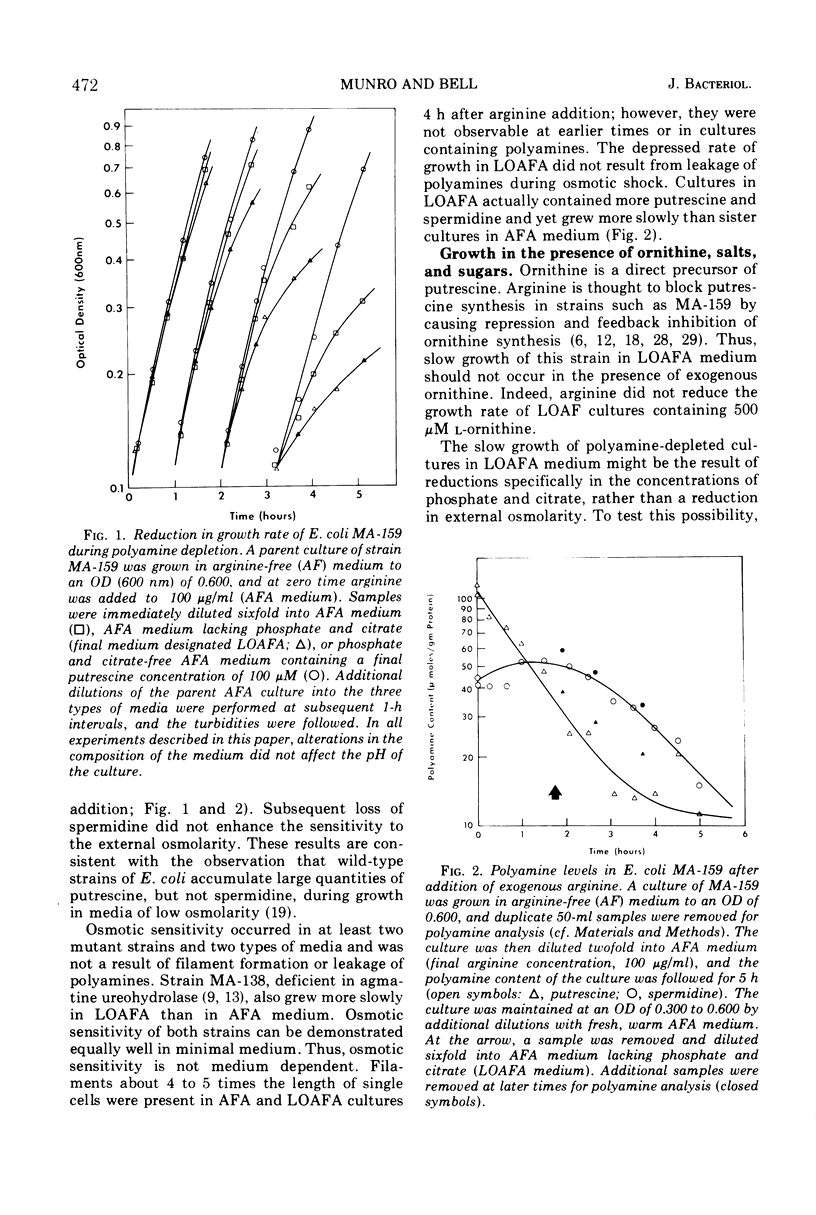
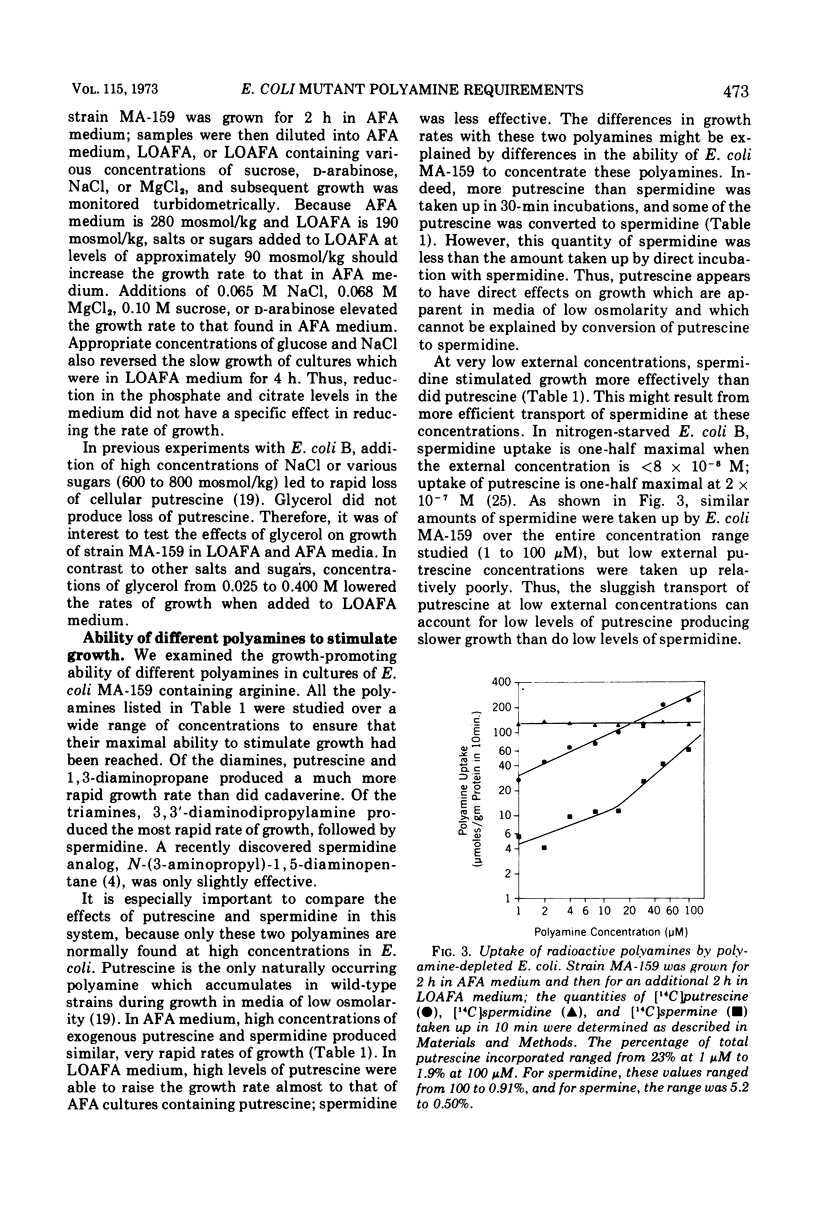
Escherichia coli MA-159 is deficient in agmatine ureohydrolase. After addition of exogenous arginine, the cellular putrescine content declines immediately and exponentially; however, the spermidine content remains normal for 3 h. The growth rate of such cultures, measured turbidometrically, slows gradually over many hours. Putrescine-depleted cultures grow especially slowly in media of low osmolarity, whereas nondepleted cultures grow at similar and rapid rates in media of either normal or low osmolarity. External osmolarity also affects the ability of various exogenous polyamines to stimulate growth of putrescine-depleted cultures. In medium of normal osmolarity, putrescine and spermidine both allow sustained rapid growth for many hours. In low osmolarity medium, putrescine allows sustained rapid growth, whereas cultures containing spermidine grow more slowly; this result cannot be explained by conversion of putrescine to spermidine, for cultures grown with exogenous putrescine contain smaller spermidine pools than do cultures grown with exogenous spermidine. Spermine greatly stimulates growth in medium of normal osmolarity; however, in medium of low osmolarity, spermine is much less effective and can block the action of putrescine. Several other polyamines have been studied in this system. These results confirm and expand previous reports that polyamines are necessary for growth of E. coli and suggest that putrescine may have a specific function during growth in media of low osmolarity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERTON J. I., LOCKE D. J. Extraction of pigment from cooked cured-meat products. Nature. 1955 May 7;175(4462):818–819. doi: 10.1038/175818b0. [DOI] [PubMed] [Google Scholar]
- Bachrach U. Metabolism and function of spermine and related polyamines. Annu Rev Microbiol. 1970;24:109–134. doi: 10.1146/annurev.mi.24.100170.000545. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBIN D. T., ROSENTHAL S. M. The acetylation of polyamines in Escherichia coli. J Biol Chem. 1960 Mar;235:776–782. [PubMed] [Google Scholar]
- Dion A. S., Cohen S. S. Polyamines in the synthesis of bacteriophage deoxyribonucleic acid. II. Requirement for polyamines in T4 infection of a polyamine auxotroph. J Virol. 1972 Mar;9(3):423–430. doi: 10.1128/jvi.9.3.423-430.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORINI L., GUNDERSEN W., BURGER M. Genetics of regulation of enzyme synthesis in the arginine biosynthetic pathway of Escherichia coli. Cold Spring Harb Symp Quant Biol. 1961;26:173–182. doi: 10.1101/sqb.1961.026.01.022. [DOI] [PubMed] [Google Scholar]
- HERBST E. J., SNELL E. E. Putrescine and related compounds as growth factors for Hemophilus parainfluenzae 7991. J Biol Chem. 1949 Nov;181(1):47–54. [PubMed] [Google Scholar]
- Hirshfield I. N., Rosenfeld H. J., Leifer Z., Maas W. K. Isolation and characterization of a mutant of Escherichia coli blocked in the synthesis of putrescine. J Bacteriol. 1970 Mar;101(3):725–730. doi: 10.1128/jb.101.3.725-730.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara H., Snell E. E. SPERMINE AND RELATED POLYAMINES AS GROWTH STIMULANTS FOR Lactobacillus Casei. Proc Natl Acad Sci U S A. 1957 Oct 15;43(10):867–871. doi: 10.1073/pnas.43.10.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAAS W. K. Studies on repression of arginine biosynthesis in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1961;26:183–191. doi: 10.1101/sqb.1961.026.01.023. [DOI] [PubMed] [Google Scholar]
- MAGER J. Influence of osmotic pressure on the polyamine requirement of Neisseria perflava and Pasteurella tularensis for growth in defined media. Nature. 1955 Nov 12;176(4489):933–934. doi: 10.1038/176933a0. [DOI] [PubMed] [Google Scholar]
- MAGER J. Spermine as a protective agent against osmotic lysis. Nature. 1959 Jun 27;183:1827–1828. doi: 10.1038/1831827a0. [DOI] [PubMed] [Google Scholar]
- MARTIN W. H., Jr, PELCZAR M. J., Jr, HANSEN P. A. Putrescine as a growth requirement for Neisseria. Science. 1952 Oct 31;116(3018):483–484. doi: 10.1126/science.116.3018.483. [DOI] [PubMed] [Google Scholar]
- Mills J., Dubin D. T. Some effects of spermine on Escherichia coli. Mol Pharmacol. 1966 Jul;2(4):311–318. [PubMed] [Google Scholar]
- Morris D. R., Jorstad C. M. Isolation of conditionally putrescine-deficient mutants of Escherichia coli. J Bacteriol. 1970 Mar;101(3):731–737. doi: 10.1128/jb.101.3.731-737.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro G. F., Hercules K., Morgan J., Sauerbier W. Dependence of the putrescine content of Escherichia coli on the osmotic strength of the medium. J Biol Chem. 1972 Feb 25;247(4):1272–1280. [PubMed] [Google Scholar]
- NOVICK R. P., MAAS W. K. Control by endogenously synthesized arginine of the formation of ornithine transcarbamylase in Escherichia coli. J Bacteriol. 1961 Feb;81:236–240. doi: 10.1128/jb.81.2.236-240.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens L. The biochemical role of naturally occurring polyamines in nucleic acid synthesis. Biol Rev Camb Philos Soc. 1970 Feb;45(1):1–27. doi: 10.1111/j.1469-185x.1970.tb01073.x. [DOI] [PubMed] [Google Scholar]
- TRAUB A., MAGER J., GROSSOWICZ N. Studies on the nutrition of Pasteurella tularensis. J Bacteriol. 1955 Jul;70(1):60–69. doi: 10.1128/jb.70.1.60-69.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W., Dobbs L. G. Metabolism of 1,4-diaminobutane and spermidine in Escherichia coli: the effects of low temperature during storage and harvesting of cultures. J Biol Chem. 1970 Apr 25;245(8):2086–2091. [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Transport systems for 1,4-diaminobutane, spermidine, and spermine in Escherichia coli. J Biol Chem. 1966 Aug 25;241(16):3714–3723. [PubMed] [Google Scholar]
- Tabor C. W. The effects of temperature on the acetylation of spermidine. Biochem Biophys Res Commun. 1968 Feb 26;30(4):339–342. doi: 10.1016/0006-291x(68)90747-x. [DOI] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Biosynthesis and metabolism of 1,4-diaminobutane, spermidine, spermine, and related amines. Adv Enzymol Relat Areas Mol Biol. 1972;36:203–268. doi: 10.1002/9780470122815.ch7. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J. Aspects of repression in the regulation of enzyme synthesis: pathway-wide control and enzyme-specific response. Cold Spring Harb Symp Quant Biol. 1961;26:163–172. doi: 10.1101/sqb.1961.026.01.021. [DOI] [PubMed] [Google Scholar]
- VYAS S., MAAS W. K. Feedback inhibition of acetylglutamate synthetase by arginine in Escherichia coli. Arch Biochem Biophys. 1963 Mar;100:542–546. doi: 10.1016/0003-9861(63)90124-3. [DOI] [PubMed] [Google Scholar]
- Young D. V., Srinivasan P. R. Regulation of macromolecular synthesis by putrescine in a conditional Escherichia coli putrescine auxotroph. J Bacteriol. 1972 Oct;112(1):30–39. doi: 10.1128/jb.112.1.30-39.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



