Abstract
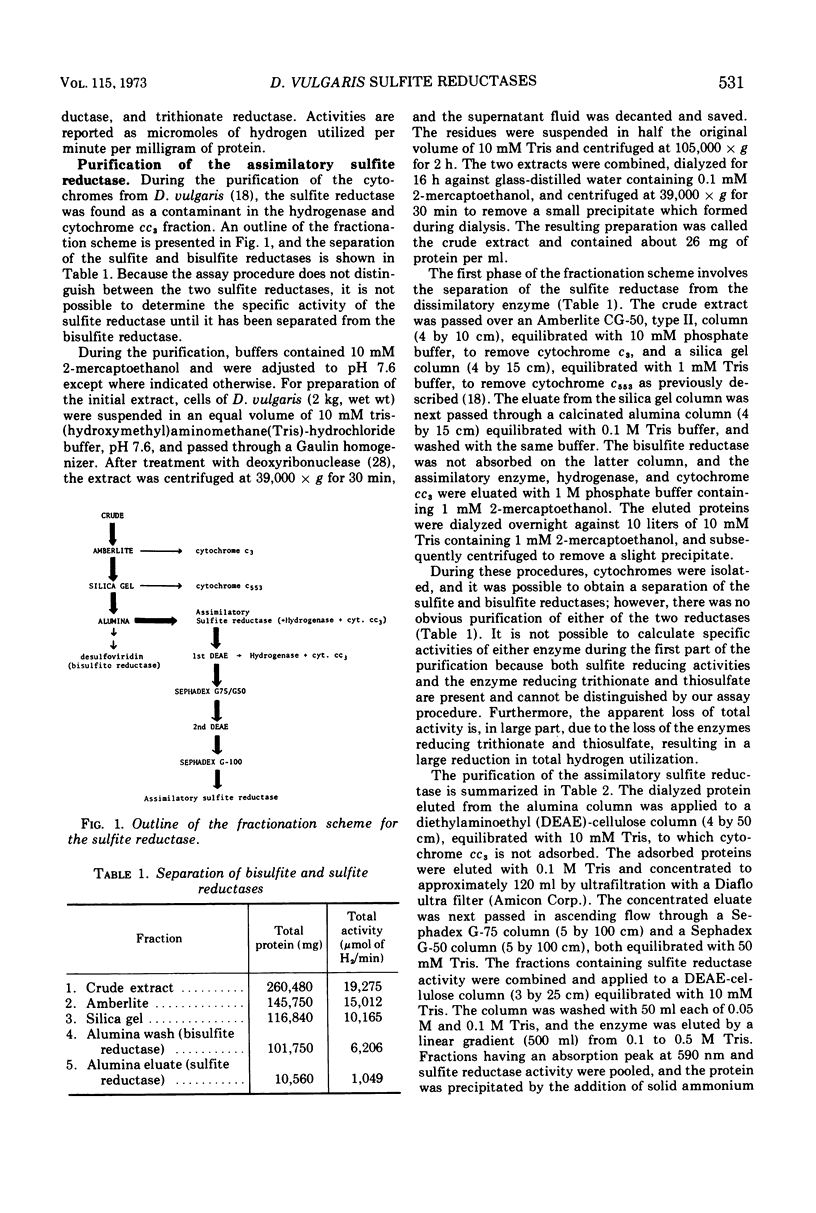
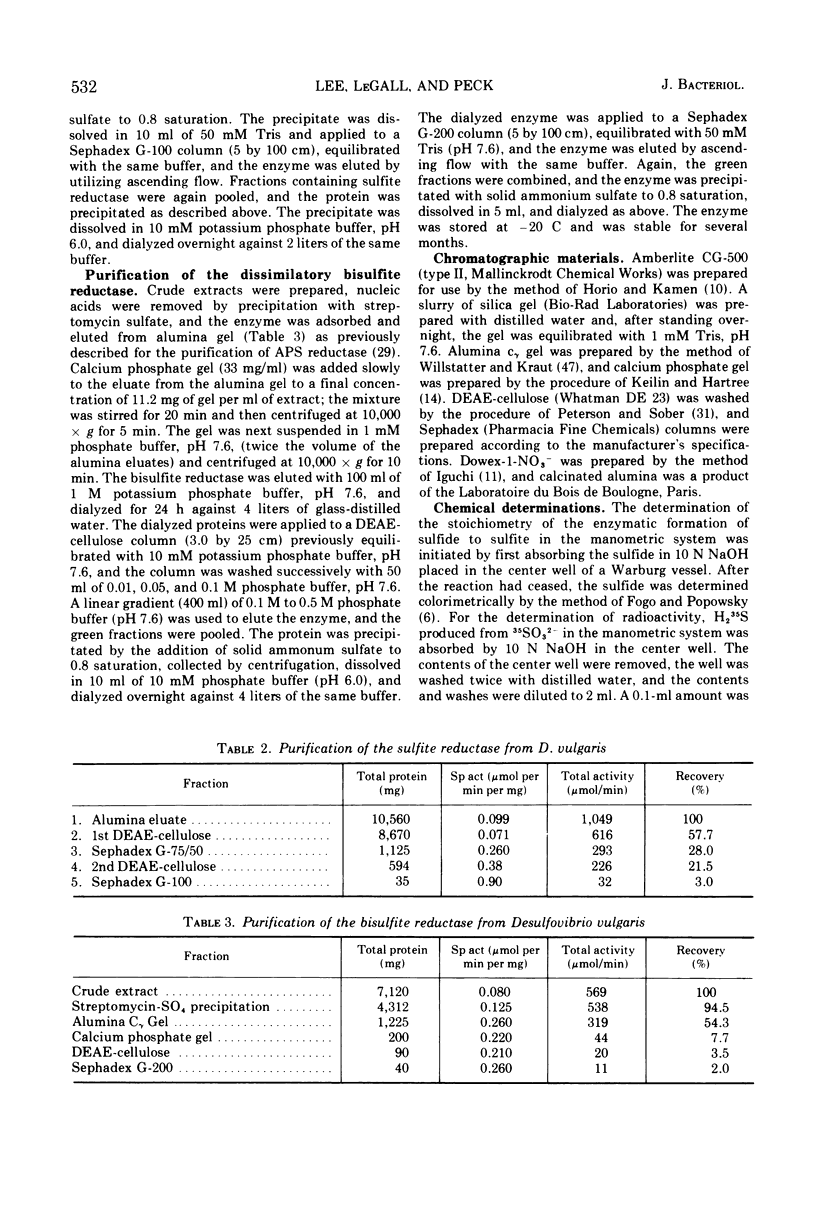
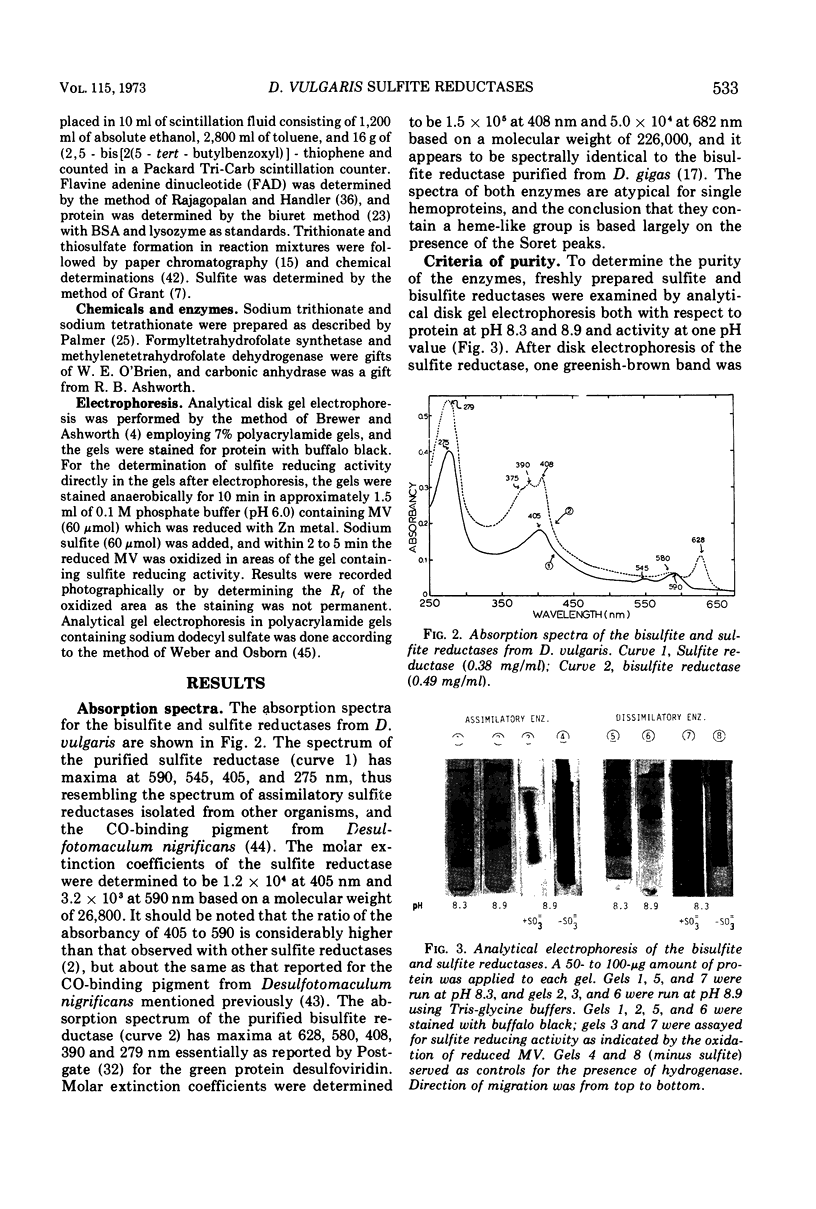
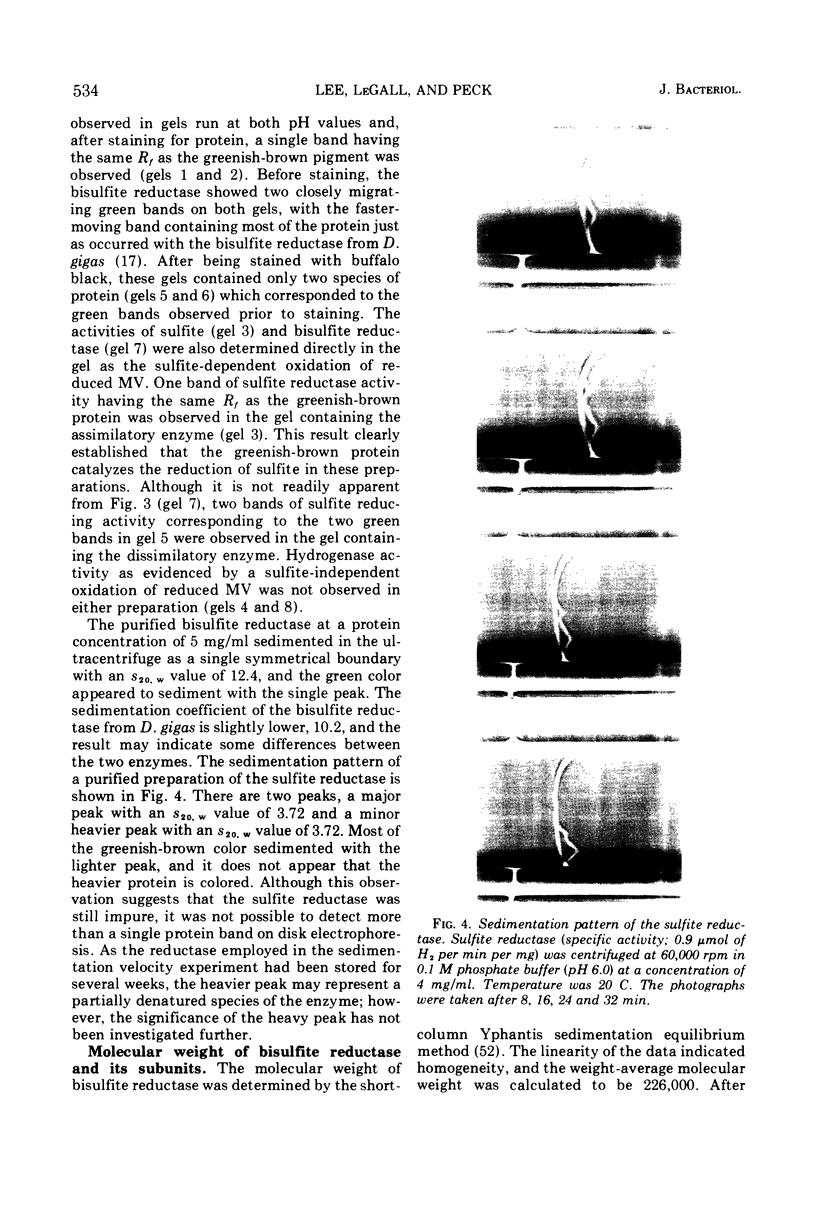
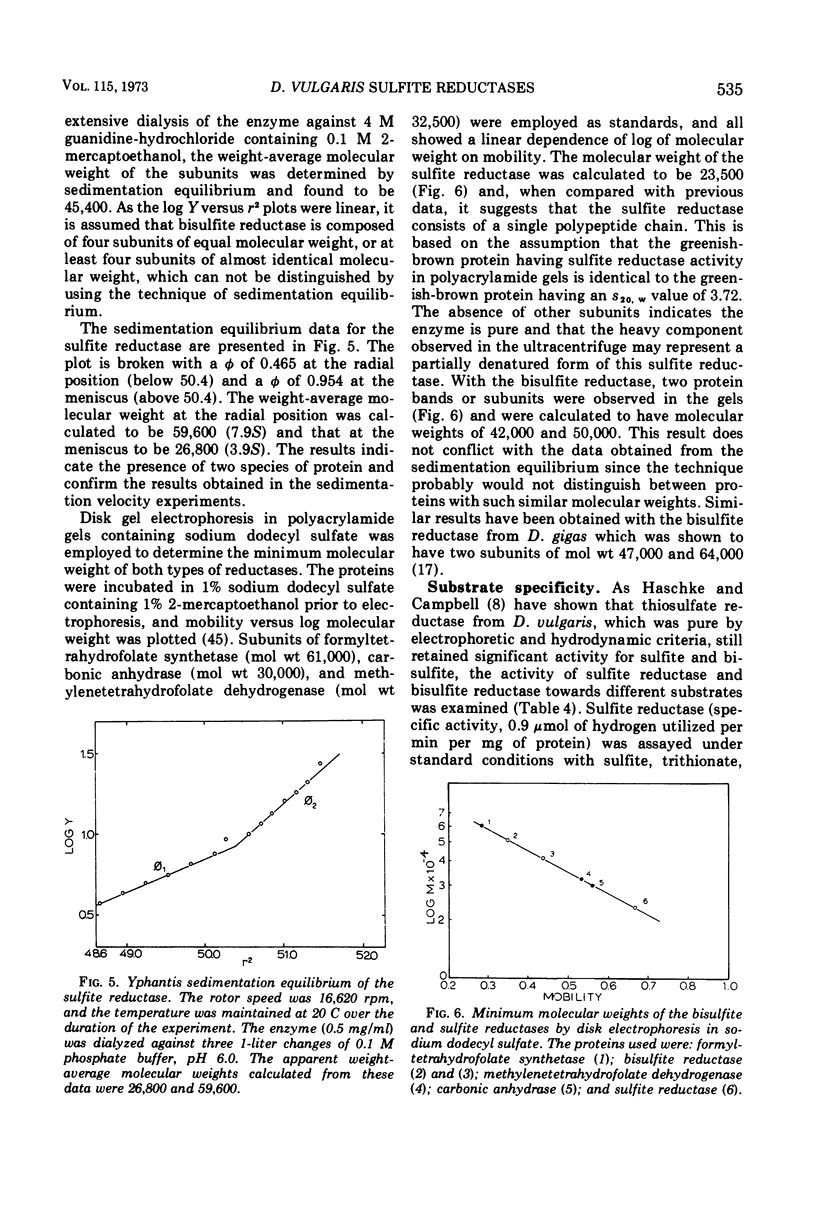
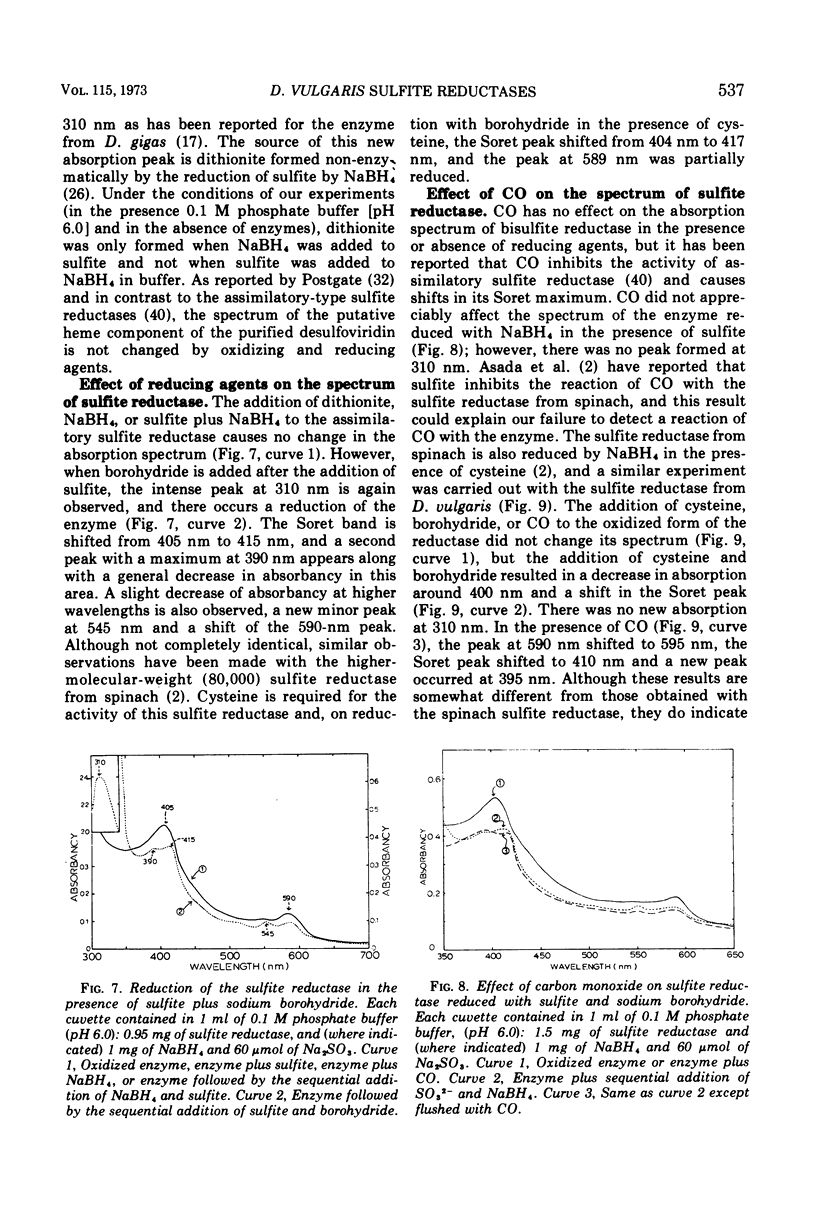
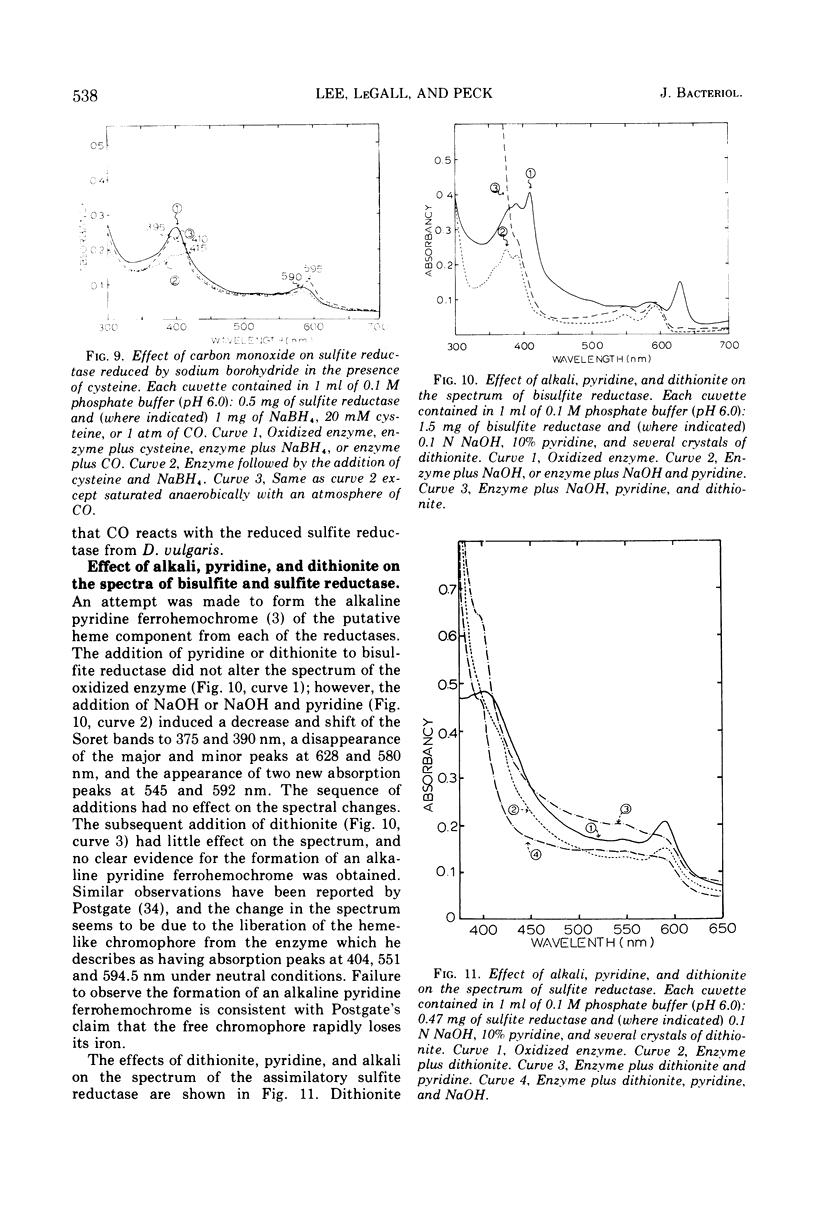
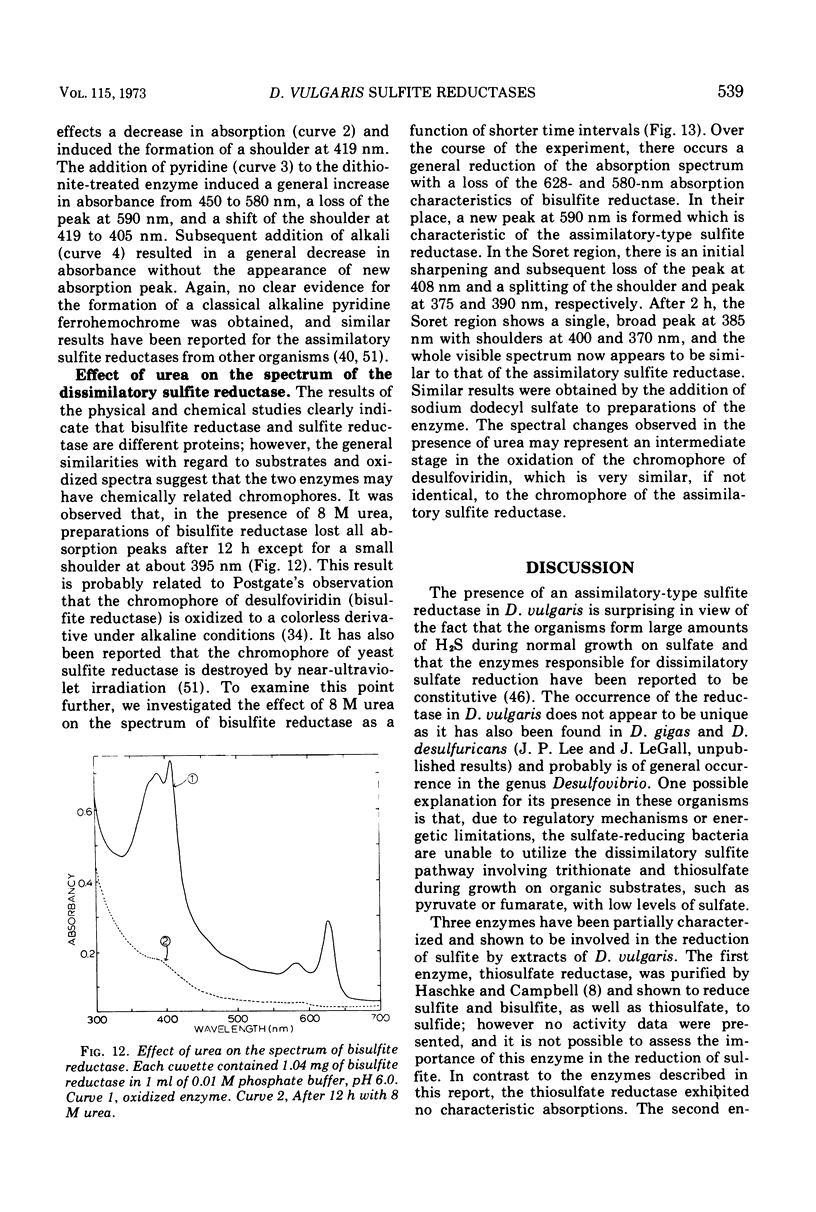
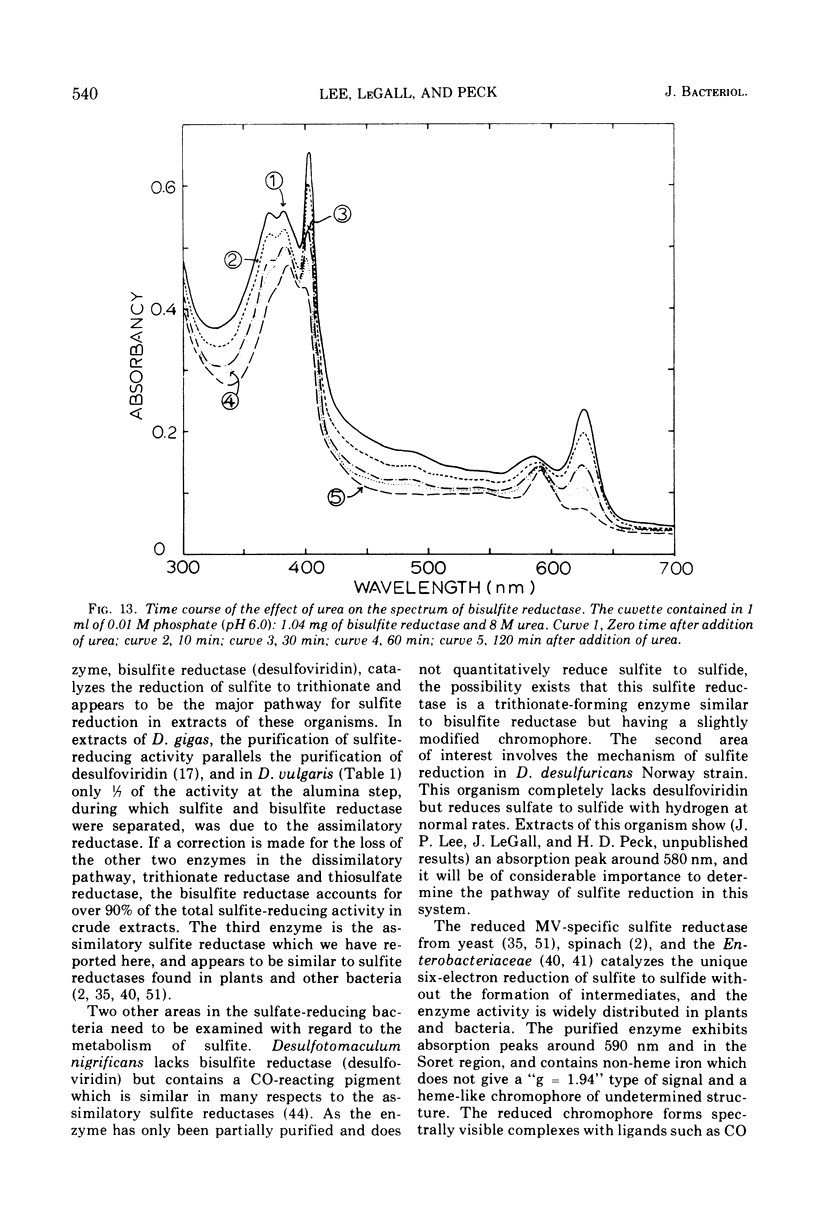
Bisulfite reductase (desulfoviridin) and an assimilatory sulfite reductase have been purified from extracts of Desulfovibrio vulgaris. The bisulfite reductase has absorption maxima at 628, 580, 408, 390, and 279 nm, and a molecular weight of 226,000 by sedimentation equilibrium, and was judged to be free of other proteins by disk electrophoresis and ultracentrifugation. On gels, purified bisulfite reductase exhibited two green bands which coincided with activity and protein. The enzyme appears to be a tetramer but was shown to have two different types of subunits having molecular weights of 42,000 and 50,000. The chromophore did not form an alkaline ferrohemochromogen, was not reduced with dithionite or borohydride, and did not form a spectrally visible complex with CO. The assimilatory sulfite reductase has absorption maxima at 590, 545, 405 and 275 nm and a molecular weight of 26,800, and appears to consist of a single polypeptide chain as it is not dissociated into subunits by sodium dodecyl sulfate. By disk electrophoresis, purified sulfite reductase exhibited a single greenish-brown band which coincided with activity and protein. The sole product of the reduction was sulfide, and the chromophore was reduced by borohydride in the presence of sulfite. Carbon monoxide reacted with the reduced chromophore but it did not form a typical pyridine ferrohemochromogen. Thiosulfate, trithionate, and tetrathionate were not reduced by either enzyme preparation. In the presence of 8 M urea, the spectrum of bisulfite reductase resembles that of the sulfite reductase, thus suggesting a chemical relationship between the two chromophores.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asada K., Tamura G., Bandurski R. S. Methyl viologen-linked sulfite reductase from spinach leaves. J Biol Chem. 1969 Sep 25;244(18):4904–4915. [PubMed] [Google Scholar]
- Brewer J. M., Ashworth R. B. Disc electrophoresis. J Chem Educ. 1969 Jan;46(1):41–45. doi: 10.1021/ed046p41. [DOI] [PubMed] [Google Scholar]
- Findley J. E., Akagi J. M. Role of thiosulfate in bisulfite reduction as catalyzed by Desulfovibrio vulgaris. J Bacteriol. 1970 Sep;103(3):741–744. doi: 10.1128/jb.103.3.741-744.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORIO T., KAMEN M. D. Preparation and properties of three pure crystalline bacterial haem proteins. Biochim Biophys Acta. 1961 Apr 1;48:266–286. doi: 10.1016/0006-3002(61)90476-0. [DOI] [PubMed] [Google Scholar]
- Haschke R. H., Campbell L. L. Thiosulfate reductase of Desulfovibrio vulgaris. J Bacteriol. 1971 May;106(2):603–607. doi: 10.1128/jb.106.2.603-607.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatchikian E. C., Le Gall J., Bruschi M., Dubourdieu M. Regulation of the reduction of sulfite and thiosulfate by ferredoxin, flavodoxin and cytochrome cc' 3 in extracts of the sulfate reducer Desulfovibrio gigas. Biochim Biophys Acta. 1972 Mar 8;258(3):701–708. doi: 10.1016/0005-2744(72)90171-4. [DOI] [PubMed] [Google Scholar]
- ISHIMOTO M., YAGI T. Biochemical studies on sulfate-reducing bacteria. IX. Sulfite reductase. J Biochem. 1961 Feb;49:103–109. doi: 10.1093/oxfordjournals.jbchem.a127264. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Tachibana S., Ishimoto M. Intermediary formation of trithionate in sulfite reduction by a sulfate-reducing bacterium. J Biochem. 1969 Jan;65(1):155–157. [PubMed] [Google Scholar]
- Kobayashi K., Takahashi E., Ishimoto M. Biochemical studies on sulfate-reducing bacteria. XI. Purification and some properties of sulfite reductase, desulfoviridin. J Biochem. 1972 Oct;72(4):879–887. doi: 10.1093/oxfordjournals.jbchem.a129982. [DOI] [PubMed] [Google Scholar]
- LEGALL J., MAZZA G., DRAGONI N. LE CYTOCHROME C3 DE DESULFOVIBRIO GIGAS. Biochim Biophys Acta. 1965 May 18;99:385–387. [PubMed] [Google Scholar]
- LEVIN R., BRAUER R. W. The biuret reaction for the determination of proteins; an improved reagent and its application. J Lab Clin Med. 1951 Sep;38(3):474–480. [PubMed] [Google Scholar]
- Le Gall J., Bruschi-Heriaud M., DerVartanian D. V. Electron paramagnetic resonance and light absorption studies on c-type cytochromes of the anaerobic sulfate reducer Desulfovibrio. Biochim Biophys Acta. 1971 Jun 15;234(3):499–512. doi: 10.1016/0005-2728(71)90216-7. [DOI] [PubMed] [Google Scholar]
- Le Gall J., Dragoni N. Dependance of sulfite reduction on a crystallized ferredoxin from Desulfovibrio gigas. Biochem Biophys Res Commun. 1966 Apr 19;23(2):145–149. doi: 10.1016/0006-291x(66)90519-5. [DOI] [PubMed] [Google Scholar]
- Lee J. P., Peck H. D., Jr Purification of the enzyme reducing bisulfite to trithionate from Desulfovibrio gigas and its identification as desulfoviridin. Biochem Biophys Res Commun. 1971 Nov 5;45(3):583–589. doi: 10.1016/0006-291x(71)90457-8. [DOI] [PubMed] [Google Scholar]
- Legall J., DerVartanian D. V., Spilker E., Lee J. P., Peck H. D., Jr Evidence for the involvement of non-heme iron in the active site of hydrogenase from Desulfovibrio vulgaris. Biochim Biophys Acta. 1971 Jun 15;234(3):526–530. [PubMed] [Google Scholar]
- MILLER J. D., SALEH A. M. A SULPHATE-REDUCING BACTERIUM CONTAINING CYTOCHROME C3 BUT LACKING DESULFOVIRIDIN. J Gen Microbiol. 1964 Dec;37:419–423. doi: 10.1099/00221287-37-3-419. [DOI] [PubMed] [Google Scholar]
- PECK H. D., Jr, DEACON T. E., DAVIDSON J. T. STUDIES ON ADENOSINE 5'-PHOSPHOSULFATE REDUCTASE FROM DESULFOVIBRIO DESULFURICANS AND THIOBACILLUS THIOPARUS. I. THE ASSAY AND PURIFICATION. Biochim Biophys Acta. 1965 Mar 22;96:429–446. doi: 10.1016/0005-2787(65)90561-7. [DOI] [PubMed] [Google Scholar]
- PECK H. D., Jr, GEST H. A new procedure for assay of bacterial hydrogenases. J Bacteriol. 1956 Jan;71(1):70–80. doi: 10.1128/jb.71.1.70-80.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PECK H. D., Jr The role of adenosine-5'-phosphosulfate in the reduction of sulfate to sulfite by Desulfovibrio desulfuricans. J Biol Chem. 1962 Jan;237:198–203. [PubMed] [Google Scholar]
- POSTGATE J. R. Cytochrome c3 and desulphoviridin; pigments of the anaerobe Desulphovibrio desulphuricans. J Gen Microbiol. 1956 Jul;14(3):545–572. doi: 10.1099/00221287-14-3-545. [DOI] [PubMed] [Google Scholar]
- POSTGATE J. A diagnostic reaction of Desulphovibrio desulphuricans. Nature. 1959 Feb 14;183(4659):481–482. doi: 10.1038/183481b0. [DOI] [PubMed] [Google Scholar]
- Peck H. D., Jr Phosphorylation coupled with electron transfer in extracts of the sulfate reducing bacterium, Desulfovibrio gigas. Biochem Biophys Res Commun. 1966 Jan 4;22(1):112–118. doi: 10.1016/0006-291x(66)90611-5. [DOI] [PubMed] [Google Scholar]
- Prabhakararao K., Nicholas D. J. Sulphite reductase from bakers' yeast: a haemoflavoprotein. Biochim Biophys Acta. 1969 Jun 24;180(2):253–263. doi: 10.1016/0005-2728(69)90112-1. [DOI] [PubMed] [Google Scholar]
- RAJAGOPALAN K. V., HANDLER P. THE ABSORPTION SPECTRA OF IRON-FLAVOPROTEINS. J Biol Chem. 1964 May;239:1509–1514. [PubMed] [Google Scholar]
- SORBO B. A colorimetric method for the determination of thiosulfate. Biochim Biophys Acta. 1957 Feb;23(2):412–416. doi: 10.1016/0006-3002(57)90346-3. [DOI] [PubMed] [Google Scholar]
- Saunders G. F., Campbell L. L., Postgate J. R. Base composition of deoxyribonucleic acid of sulfate-reducing bacteria deduced from buoyant density measurements in cesium chloride. J Bacteriol. 1964 May;87(5):1073–1078. doi: 10.1128/jb.87.5.1073-1078.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A. On the mechanism of photosynthetic sulfate reduction. An APS-sulfotransferase from Chlorella. Arch Mikrobiol. 1972;84(1):77–86. doi: 10.1007/BF00408084. [DOI] [PubMed] [Google Scholar]
- Suh B., Akagi J. M. Formation of thiosulfate from sulfite by Desulfovibrio vulgaris. J Bacteriol. 1969 Jul;99(1):210–215. doi: 10.1128/jb.99.1.210-215.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudinger P. A. Carbon monoxide-reacting pigment from Desulfotomaculum nigrificans and its possible relevance to sulfite reduction. J Bacteriol. 1970 Oct;104(1):158–170. doi: 10.1128/jb.104.1.158-170.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHELDRAKE J. F., PASTERNAK C. A. THE CONTROL OF SULPHATE ACTIVATION IN BACTERIA. Biochem J. 1965 Jul;96:276–280. doi: 10.1042/bj0960276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOLFOLK C. A. Reduction of inorganic compounds with molecular hydrogen by Micrococcus lactilyticus. II. Stoichiometry with inorganic sulfur compounds. J Bacteriol. 1962 Oct;84:659–668. doi: 10.1128/jb.84.4.659-668.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]
- Yoshimoto A., Sato R. Studies on yeast sulfite reductase. 3. Further characterization. Biochim Biophys Acta. 1970 Nov 11;220(2):190–205. doi: 10.1016/0005-2744(70)90005-7. [DOI] [PubMed] [Google Scholar]
- Yoshimoto A., Sato R. Studies on yeast sulfite reductase. II. Partial purification and properties of genetically incomplete sulfite reductases. Biochim Biophys Acta. 1968 Apr 2;153(3):576–588. doi: 10.1016/0005-2728(68)90186-2. [DOI] [PubMed] [Google Scholar]




